A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
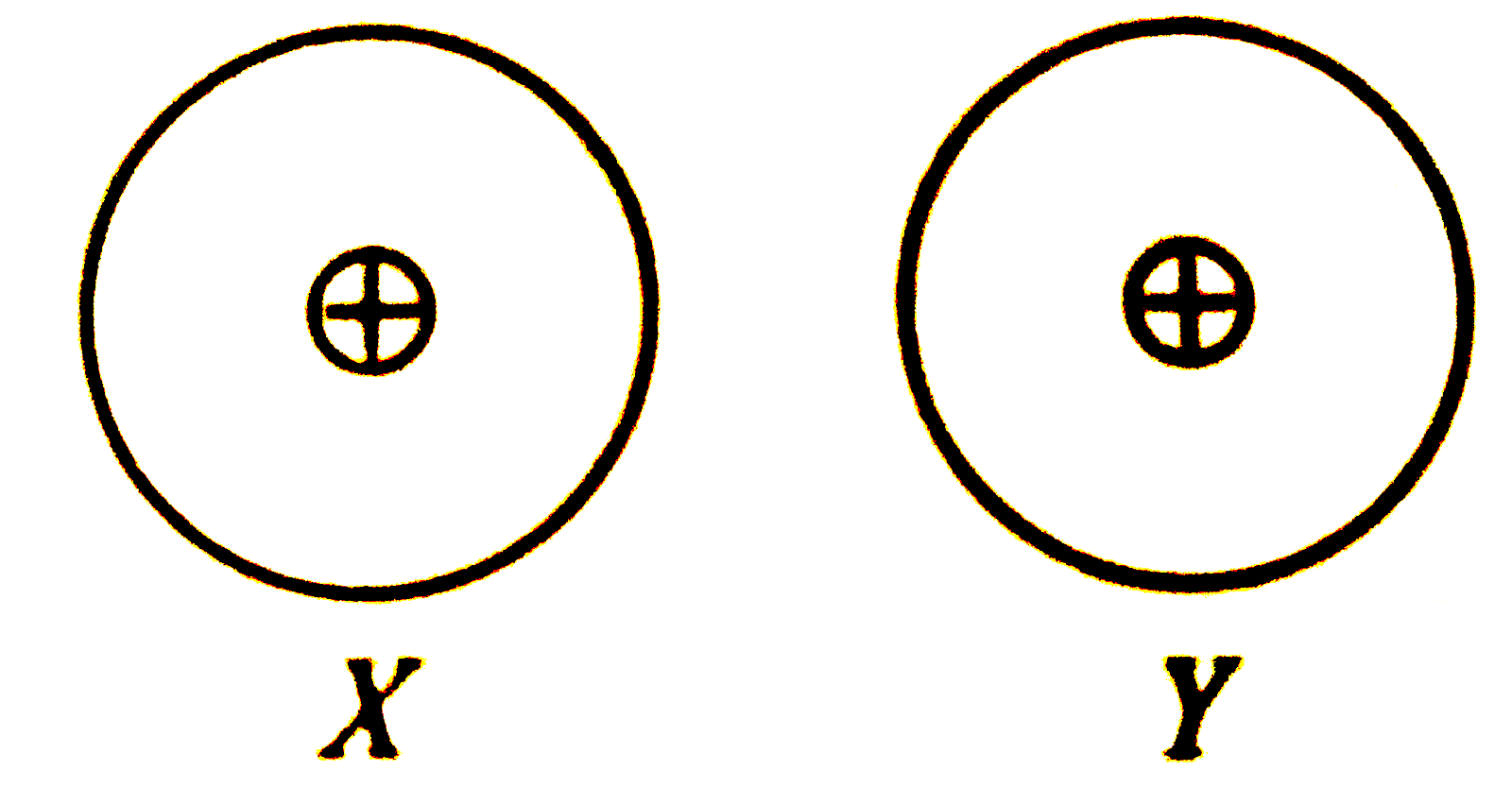
- Two atoms X and Y are non - polar and electrically symmetrical. ...
Text Solution
|
- What is meant by intermolecular space and intermolecular forces of att...
Text Solution
|
- What is intermolecular force of attraction ? Mention two types of inte...
Text Solution
|
- ' A. Intermolecular force of attraction of X gt Y B. Intermolecula...
Text Solution
|
- Two atoms X and Y are non - polar and electrically symmetrical. ...
Text Solution
|
- What is intermolecular force of attraction?
Text Solution
|
- What kind of intermolecular forces of attraction hold the molecules in...
Text Solution
|
- Which types of intermolecular force of attraction act between the mole...
Text Solution
|
- Two atoms X and Y are non-polar and electrically symmetrical What type...
Text Solution
|