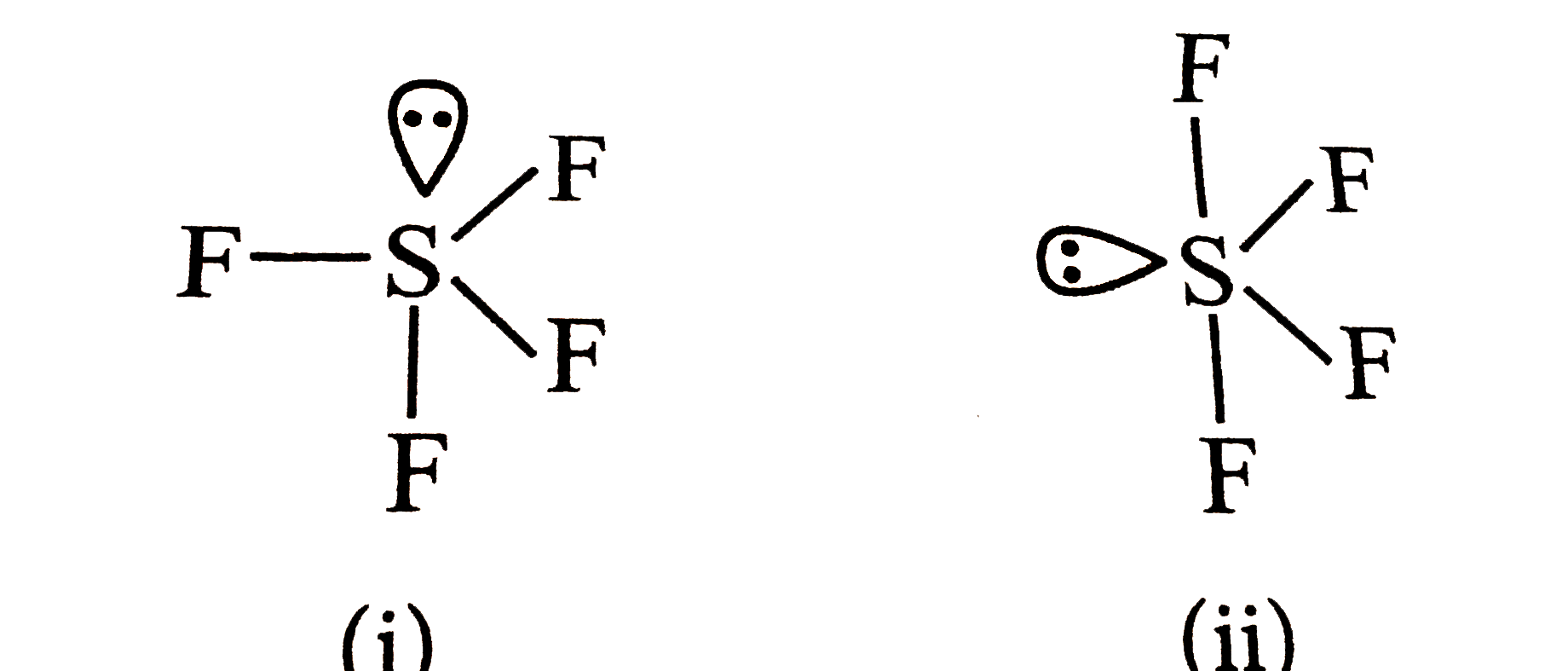A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following shapes of SF(4) is more stable and why?
Text Solution
|
- Which of the following carbocation is more stable and why?
Text Solution
|
- Which one is more stable and why?
Text Solution
|
- Predict hybridisation and shape of SF(4) molecule.
Text Solution
|
- The shapes of SF(4) and XeF(2) respectively are
Text Solution
|
- The shapes of SF(4)andXeF(2) respectively are
Text Solution
|
- SF(4) में ……… संकरण प्रयुक्त होता है तथा इसकी आकृति ……… होती है।
Text Solution
|
- Which of the following shapes of SF(4) is more stable and why?
Text Solution
|
- SF(4) has shape.
Text Solution
|