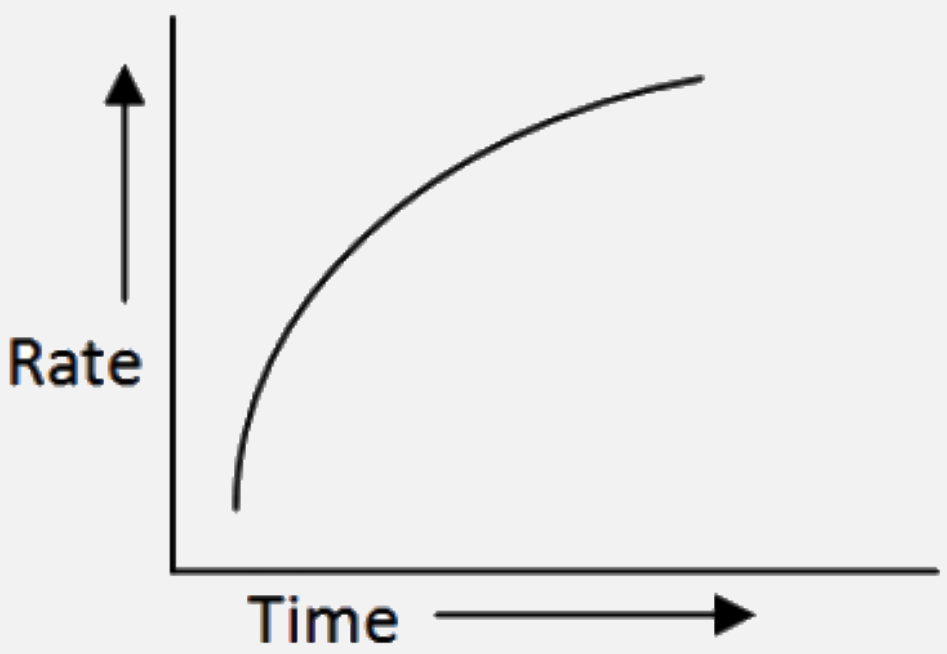
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The rate of oxidation of oxalic acid by acidified potassium permangana...
Text Solution
|
- In the titration between oxalic acid ad acidified potassium permangana...
Text Solution
|
- Which compound is oxidized by KMnO4 in presence of acid in the followi...
Text Solution
|
- Which compound is oxidized by KMnO4 in presence of acid in the followi...
Text Solution
|
- Describe the preparation of potassium permanganate. How does the acidi...
Text Solution
|
- The rate of oxidation of oxalic acid by acidified potassium permangana...
Text Solution
|
- In acid medium, potassium permanganate oxidizes oxalic acid to
Text Solution
|
- M' is the molecular weight of KMnO4 The equivalent weight of KMnO4 whe...
Text Solution
|
- The magnetic moment of autocatalyst formed in the reaction between aci...
Text Solution
|