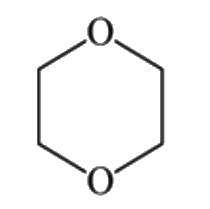
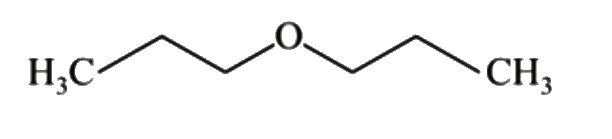
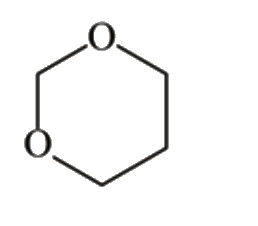
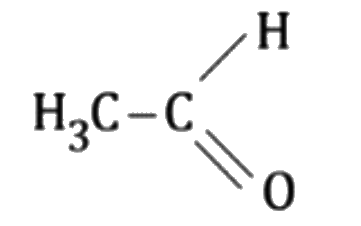
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Dehydration of ethylene glycol in presence of concentration H2SO4 will...
Text Solution
|
- H2SO4 की उपस्थिति में अल्कोहल के निर्जलीकरण की क्रियाविधि का वर्णन कीज...
Text Solution
|
- With concentrated sulphuric acid, glycol undergoes intermolecular dehy...
Text Solution
|
- Oxidation of ethylene glycol with HIO(4) gives
Text Solution
|
- Ethylene glycol is dehydrated to diethylene glycol by .
Text Solution
|
- H2SO4 के इस गुण का एक उदाहरण दीजिए निर्जलीकारक
Text Solution
|
- Dehydration of ethylene glycol in presence of concentration H2SO4 will...
Text Solution
|
- The reaction of ethylene glycol with PI3gives …………………. .
Text Solution
|
- With anhydrous ZnCl2 , ethylene glycol gives ……………………. .
Text Solution
|