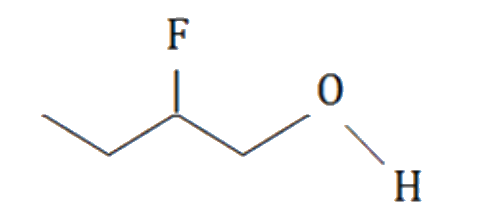
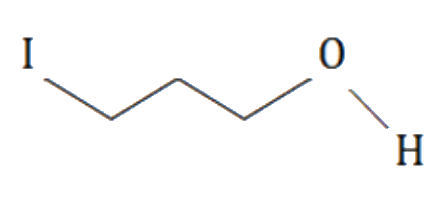
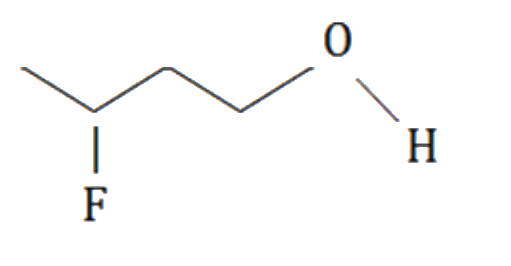
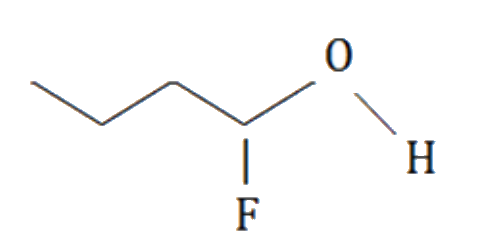
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In which of the following compounds is hydroxylic proton the most acid...
Text Solution
|
- The most acidic proton and the strongent nucleophilic nitrogen in the ...
Text Solution
|
- Which of the following has most acidic proton :
Text Solution
|
- Which one of the following compounds is not a protoric acid?
Text Solution
|
- Among the following, the compound having the most acidic proton is:
Text Solution
|
- Which of the following are hydroxyl acid .
Text Solution
|
- निम्नलिखित में कौन - सा यौगिक प्रोटॉनिक अम्ल नहीं है ?
Text Solution
|
- Which one of the following compounds is not a protoric acid?
Text Solution
|
- एक हाइड्रॉक्सिल यौगिक, जो सान्द्र हाइड्रोक्लोरिक अम्ल तथा निर्जलीय जिं...
Text Solution
|