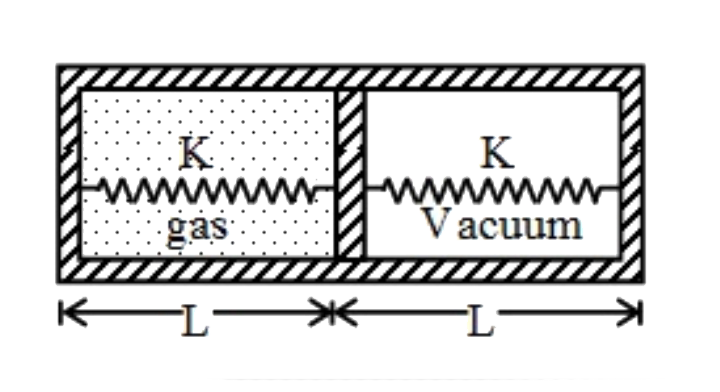
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In the given figure the area of the frictionless pistonis 1m^(2) and i...
Text Solution
|
- Under isothermal condition, a gas at 300 K expands from 0.1 L to 0.25 ...
Text Solution
|
- A fixed container is fitted with a piston which is attached to a sprin...
Text Solution
|
- Block of mass 2 m is given v(0) towards the right. If L is the natural...
Text Solution
|
- A cylinder of cross-section area. A has two pistons of negligible mass...
Text Solution
|
- When heat is supplied to the gas it expands and displaces piston by L/...
Text Solution
|
- Two blocks are connected by a spring of natural length 2 m. The force ...
Text Solution
|
- Under isothermal conditions, a gas at 300 K expands from 0.1 L to 0.25...
Text Solution
|
- वक्तव्य I बल नियतांक k = (YA)/(l), जहाँ Y यंग प्रत्यास्थता गुणांक है,...
Text Solution
|