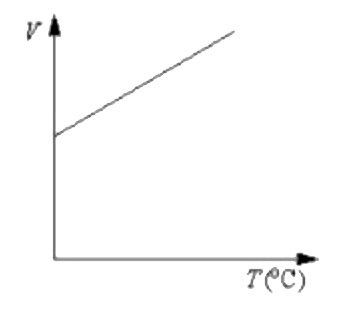
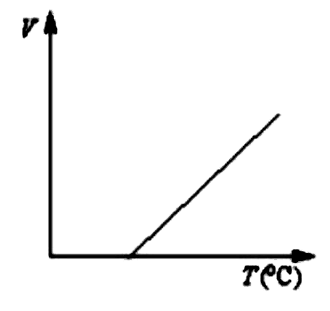
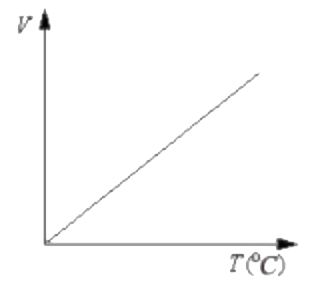
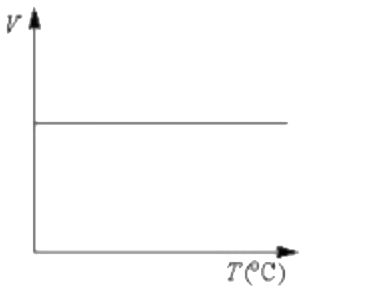
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Volume - temperature graph at atmospheric pressure for a monatomic gas...
Text Solution
|
- Which of the following volume (V)-temperature (T) plots represents the...
Text Solution
|
- आयतन (V) तथा ताप (T) के बीच खींचे गये निम्नलिखित ग्राफो में कौन-सा ग्र...
Text Solution
|
- Which of the following volume (V) - temperature (T) plots represents t...
Text Solution
|
- Which of the following volume-temperature (V-I) plots represents the b...
Text Solution
|
- Volume - temperature graph at atmospheric pressure for a monatomic gas...
Text Solution
|
- निम्न में से कौन - सा आयतन (V), ताप (T) ग्राफ ( आलेख ) , एक वायुमंडल...
Text Solution
|
- Which of the following volume (V) temperature (T) plots represents the...
Text Solution
|
- एक वायुमण्डलीय दाब पर किसी एक परमाणुक गैस के लिए आयतन (V) साथ ताप (T) ...
Text Solution
|