A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
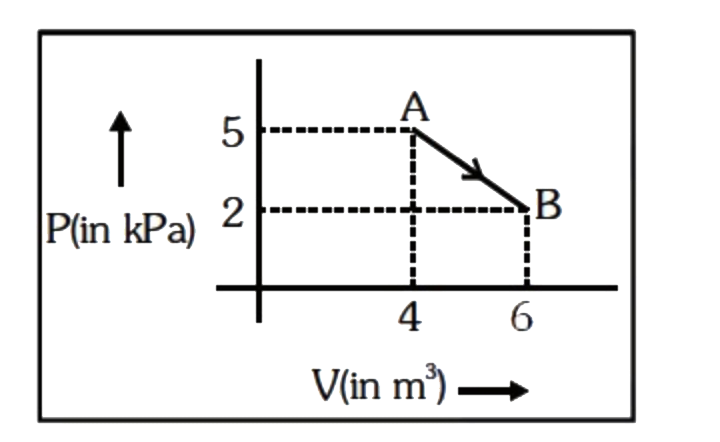
- One mole of an ideal diatomic gas undergoes a transition from A to B a...
Text Solution
|
- One mole of an ideal diatomic gas undergoes a transition from A to B a...
Text Solution
|
- One mole of an ideal monoatomic gas undergoes a cyclic process as show...
Text Solution
|
- One mole of an ideal monoatomic gas is caused to go through the cycle ...
Text Solution
|
- One mole of an ideal diatomic gas undergoes a transition from A to B a...
Text Solution
|
- One mole of an ideal diatomic gas undergoes a transition from A to B a...
Text Solution
|
- One mole of an ideal diatomic gas undergoes a transition from A to B a...
Text Solution
|
- एक आदर्श द्वि-परमाणुक गैस के 1 मोल का, AB पथ के अनुदिश, A से B टकल स...
Text Solution
|
- One mole of an ideal diatomic gas undergoes a transition from A to B a...
Text Solution
|