Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
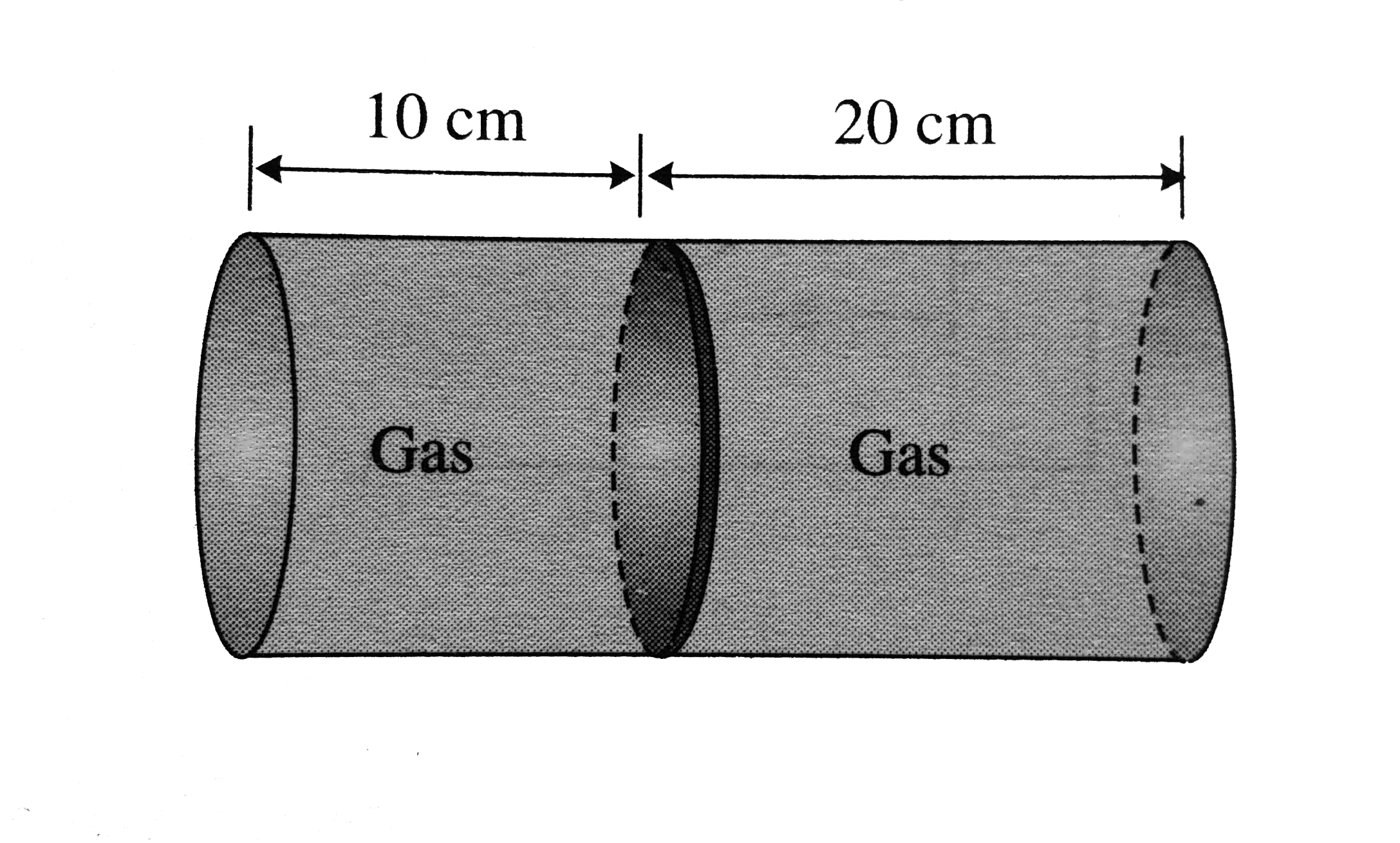
- Fig. shows a horzontal cylindrical container of length 30 cm, which is...
Text Solution
|
- Show a cylindrical tube of volume V(0) divided in two parts by a frict...
Text Solution
|
- Shows a vessel partitioned by a fixed diathermoc separator. Different ...
Text Solution
|
- Figure shows an adiabatic cylindrical tube of volume (V0) divided in t...
Text Solution
|
- Fig. shows a horzontal cylindrical container of length 30 cm , which i...
Text Solution
|
- The right and left ventricles are separated by ................. .
Text Solution
|
- Shows a vessel partitioned by a fixed diathermoc separator. Different ...
Text Solution
|
- An adiabatic vessel of total volume V is divided into two equal parts ...
Text Solution
|
- Figure shows a horizontal cylindrical container of length 30 cm, which...
Text Solution
|