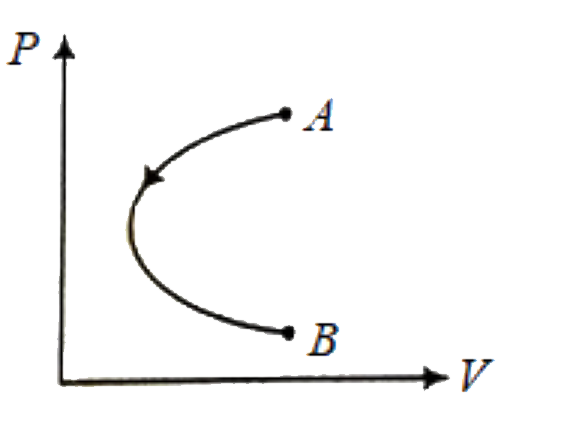
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- P - V diagram of an ideal gas is shown. The gas undergoes from initial...
Text Solution
|
- An ideal gas undergoes isothermal process from some initial state i to...
Text Solution
|
- One mole of an ideal gas goes from an initial state A to final state B...
Text Solution
|
- An ideal gas is subjected to two different process in which it is heat...
Text Solution
|
- An idel gas undrgoes isothermal process from some initial state i to f...
Text Solution
|
- एक आदर्श गैस का समान प्रारम्भिक अवस्था (P, V) से समान अन्तिम आयतन तक ए...
Text Solution
|
- An ideal gas undergoes isothermal process from some initial state i to...
Text Solution
|
- P - V diagram of an ideal gas is shown. The gas undergoes from initial...
Text Solution
|
- One mole of an ideal gas goes from an initial state A to final state B...
Text Solution
|