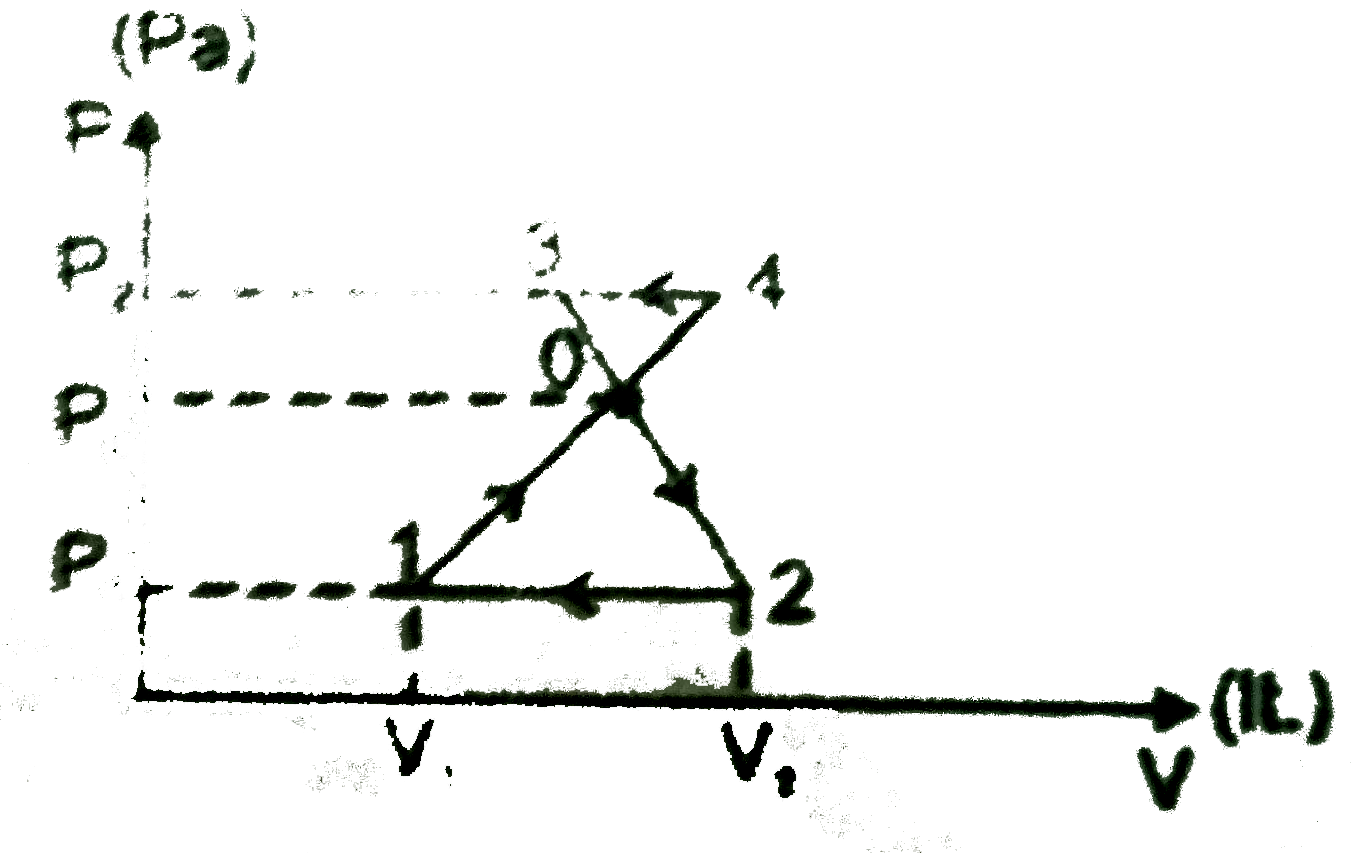
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Find the work done by an ideal gas during a closed cycle 1 rarr 4 rarr...
Text Solution
|
- A thermodynamic process is shown in the following figure. The process ...
Text Solution
|
- When temperature of a gas is 20^@C and pressure is changed from p(1)= ...
Text Solution
|
- Determine the work done by an ideal gas doing 1 rarr 4 rarr 3rarr 2 ra...
Text Solution
|
- A thermodynamic process is shown in Fig. The pressures and volumes cor...
Text Solution
|
- A thermodynamic process is shown in Fig. The pressures and volumes cor...
Text Solution
|
- Figure shows a cyclic process ABCDBEA performed on an ideal cycle. If ...
Text Solution
|
- An ideal gasa undergoes through folowing cyclic procees. 1-2 : Reversi...
Text Solution
|
- A thermodynamic process is shown in the following figure. In the proce...
Text Solution
|