A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
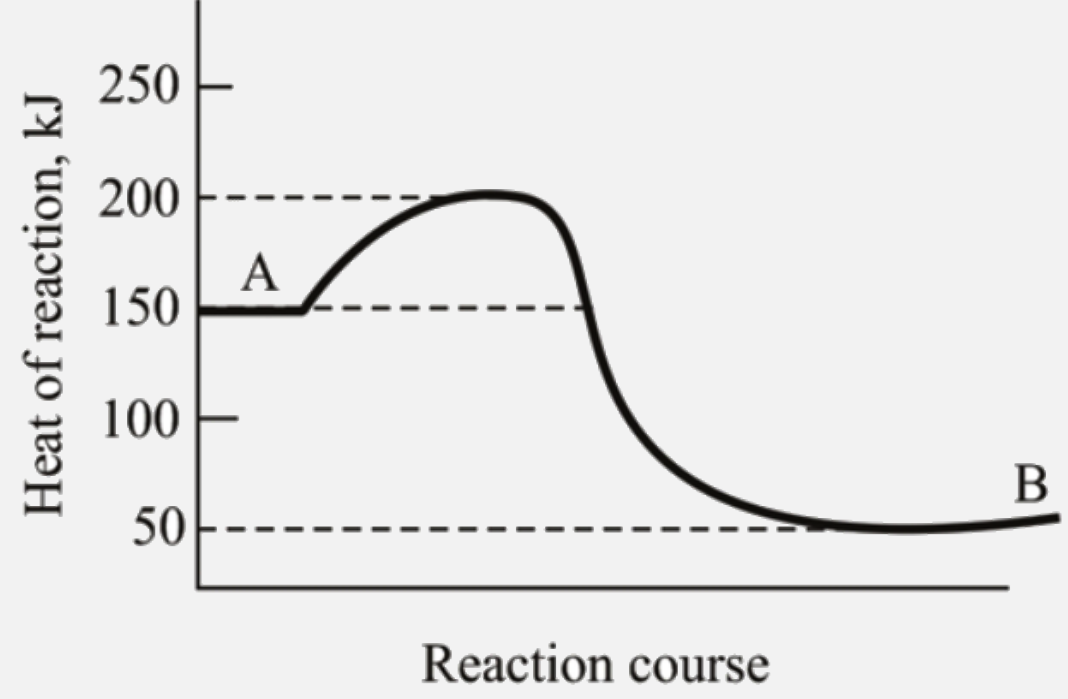
- Heat of conversion of substance A to substance B is equal to (kJ)
Text Solution
|
- The density of a substance is 400kgm^(-3) and that of another substanc...
Text Solution
|
- If 1050 kJ of heat is required to rise the temperature of 18 kg of sub...
Text Solution
|
- ऊष्मा कोई पदार्थ नहीं बल्कि "…............" है ।
Text Solution
|
- If a substance is heated or cooled, the change in mass of that substan...
Text Solution
|
- Heat of conversion of substance A to substance B is equal to (kJ)
Text Solution
|
- Molar heat capacity at constant P for a substance is equal to
Text Solution
|
- What is the conversion of a liquid substance into a solid?
Text Solution
|
- If a substance is heated or cooled, the change in mass of that substan...
Text Solution
|