A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In the compound , , the C2 - C3 bond is of the type :
Text Solution
|
- C3 চক্লে প্রথম স্থায়ী যৌগ কোন্টি ?
Text Solution
|
- In the compound , , the C2 - C3 bond is of the type :
Text Solution
|
- সংখ্যারেখা তৈরি করে নীচের সংখ্যাগুলিকে সংখ্যারেখায় দেখাও ও নাম দাও। +5...
Text Solution
|
- C3 cycle|C4 cycle#!#C2 cycle
Text Solution
|
- Which of the following compounds belong to same homo-logous series? C2...
Text Solution
|
- Most stable form of meso-2, 3-difluoro-2, 3-butandiol (across C2-C3 bo...
Text Solution
|
- In the following compound H - overset(1) C-=overset(2)C-overset(3)(CH(...
Text Solution
|
- The bond length between C2 and C3 in acrutl aldehyde is not equal to t...
Text Solution
|
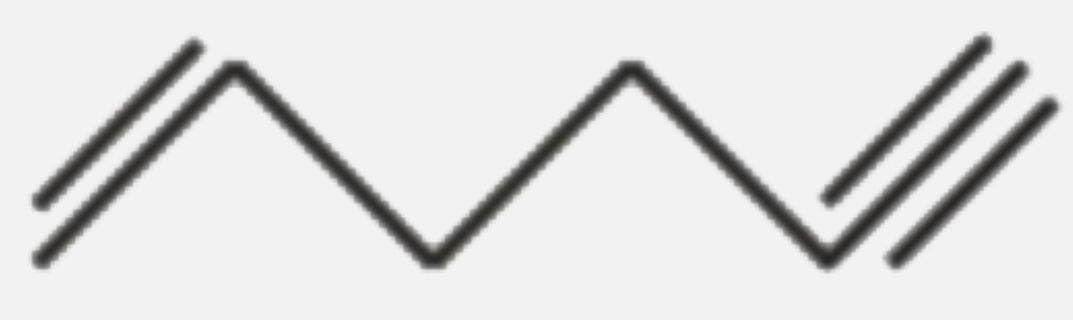 , the C2 - C3 bond is of the type :
, the C2 - C3 bond is of the type :