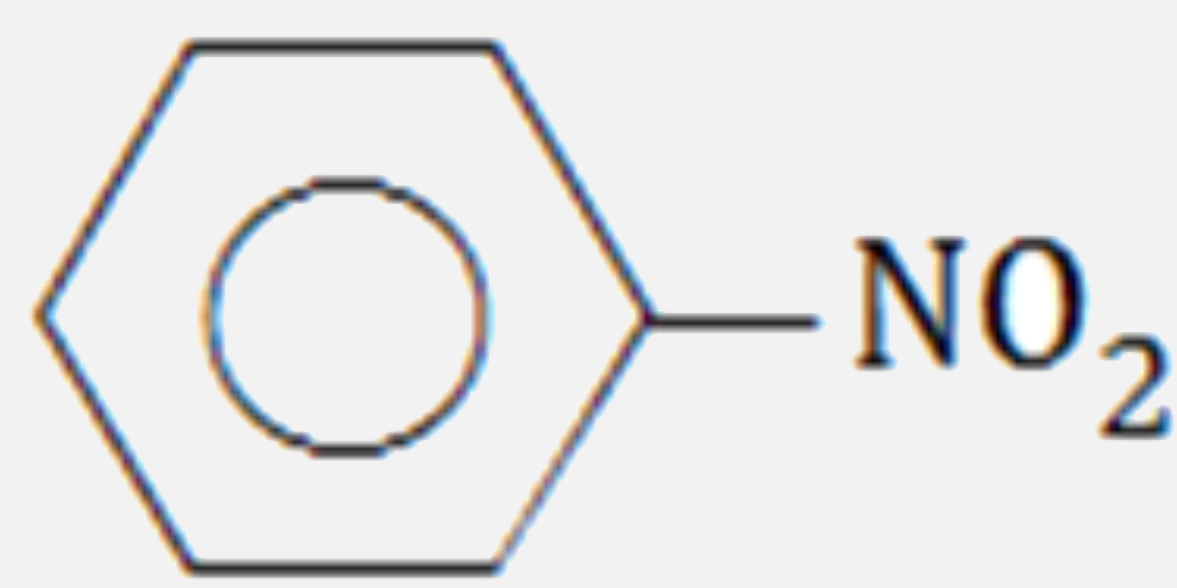
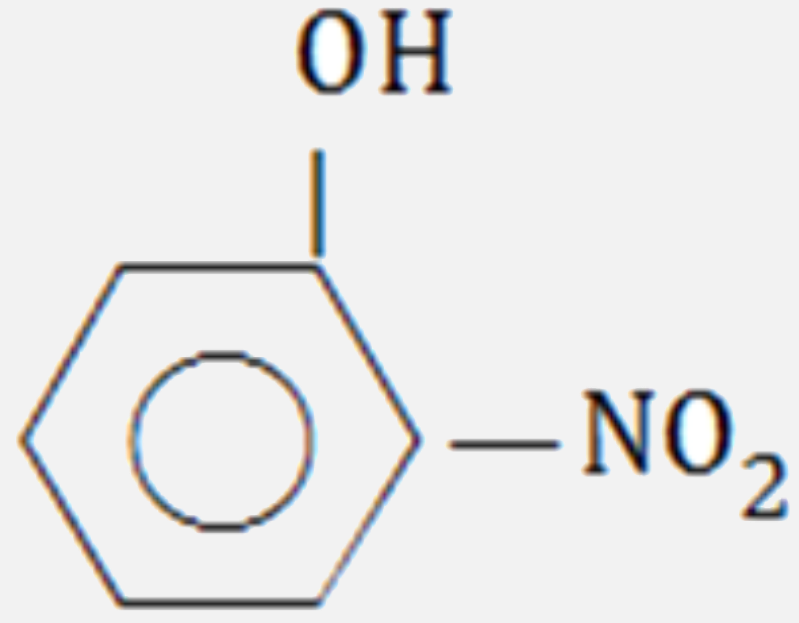
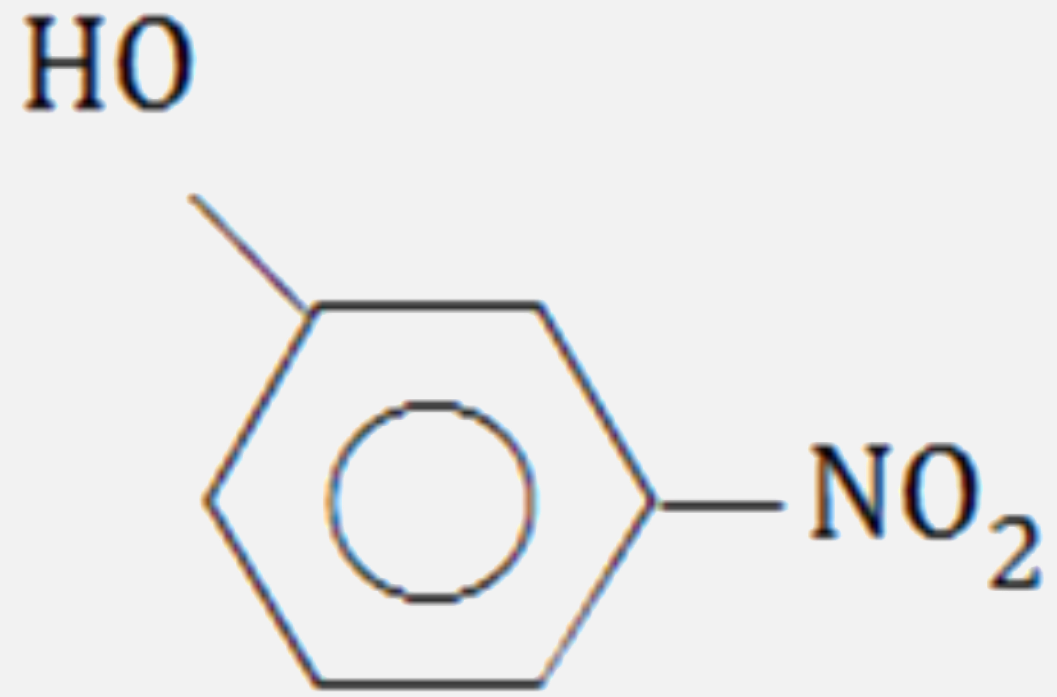
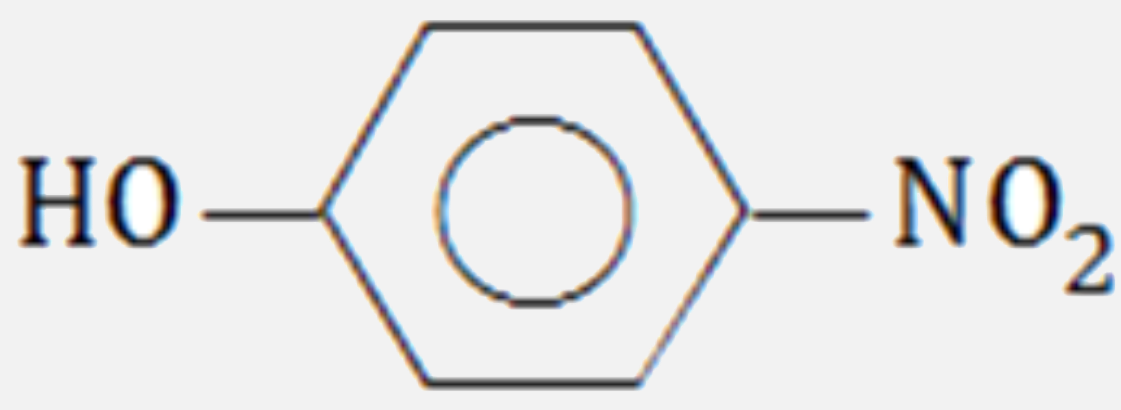
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following compounds will have the highest dipole moment ?
Text Solution
|
- Which one among the following compounds has the highest dipole moment ...
Text Solution
|
- Which compound posses highest dipole moment ?
Text Solution
|
- Which of the following halide will have highest dipole moment ?
Text Solution
|
- The compound that has the highest dipole moment is
Text Solution
|
- Which of the following compounds have finite dipole moments ?
Text Solution
|
- Which of these compounds possess highest dipole moment ?
Text Solution
|
- Which of the following will have the highest dipole moment ?
Text Solution
|
- Which of the following compounds has the highest dipole moment (D) ?
Text Solution
|