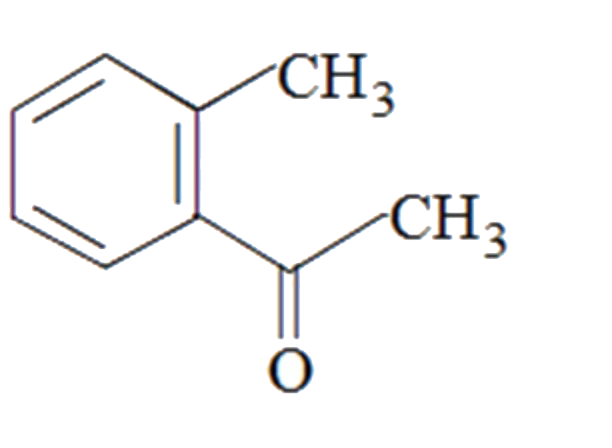
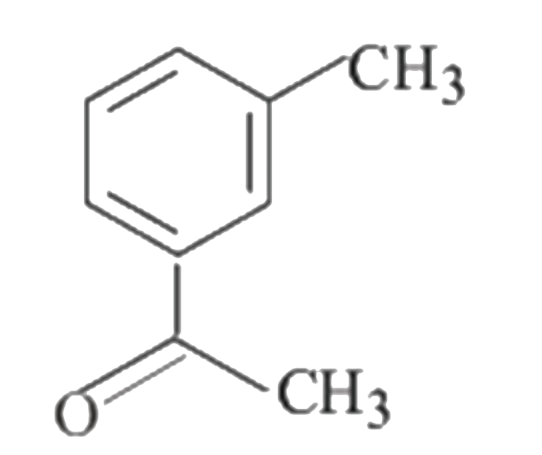
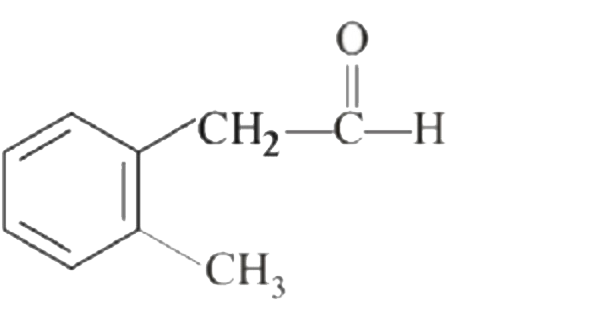
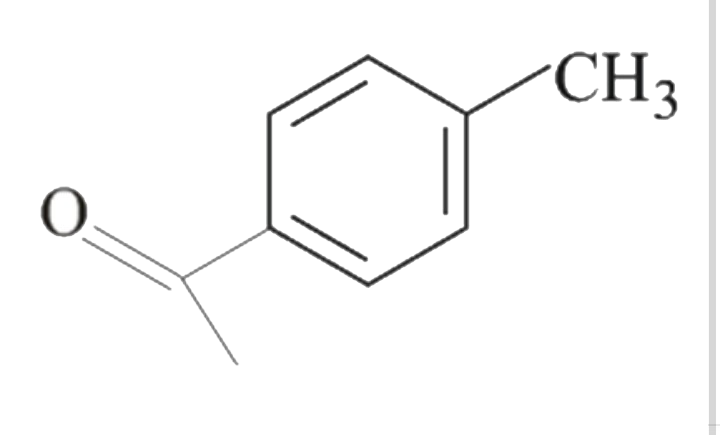
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Compound A(C(9)H(10)O) shows positive iodoform test. Oxidation of A w...
Text Solution
|
- A compound x gives iodoform test. It reacts with KMnO(4) , which on he...
Text Solution
|
- Deduct the structural formul of a compound (A) (C(10)H(16)) the gives ...
Text Solution
|
- Compound 'X' C(4)H(8)O which gives 2,4-DNP derivative and positive iod...
Text Solution
|
- A substance C(4)H(10)O yields on oxidation a compound C(4)H(8)O which ...
Text Solution
|
- A substance C(4)H(10)O yields on oxidation a compound C(4)H(8)O which ...
Text Solution
|
- An organic compound [A] C(6)H(10), on reduction first gives [B] C(6)H(...
Text Solution
|
- An unsaturated compound C(6)H(12) A) decolourless Br(2). Water and on ...
Text Solution
|
- A substance C(4)H(10)O yields on oxidation a compound C(4)H(8)O which ...
Text Solution
|