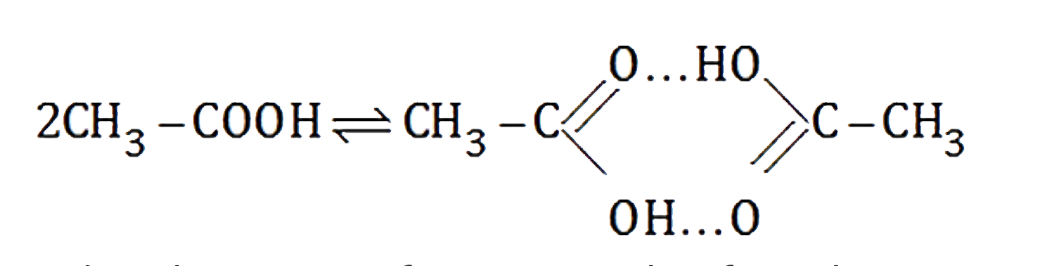A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Acetic acid undergoes dimerisation , when dissolved in benzene Mo...
Text Solution
|
- Acetic acid in the vapour state has a molecular weight of 120 because
Text Solution
|
- Acetic acid undergoes dimerisation in benzene, the van't Hoff factor i...
Text Solution
|
- Acetic acid dissolved in benzene shows a molecular mass of:
Text Solution
|
- Consider the following hypothetical equilibrium 2B(g)hArrB(2)(g) I...
Text Solution
|
- Acetic acid dimerises in benzene solution.The van't Hoff factor for th...
Text Solution
|
- Acetic acid dissolved in benzene shows a molecular mass of g...
Text Solution
|
- Acetic acid dissolved in benzene shows a molecular mass of:
Text Solution
|
- Acetic acid shows a molecular mass of 120 when dissolved in benzene. T...
Text Solution
|