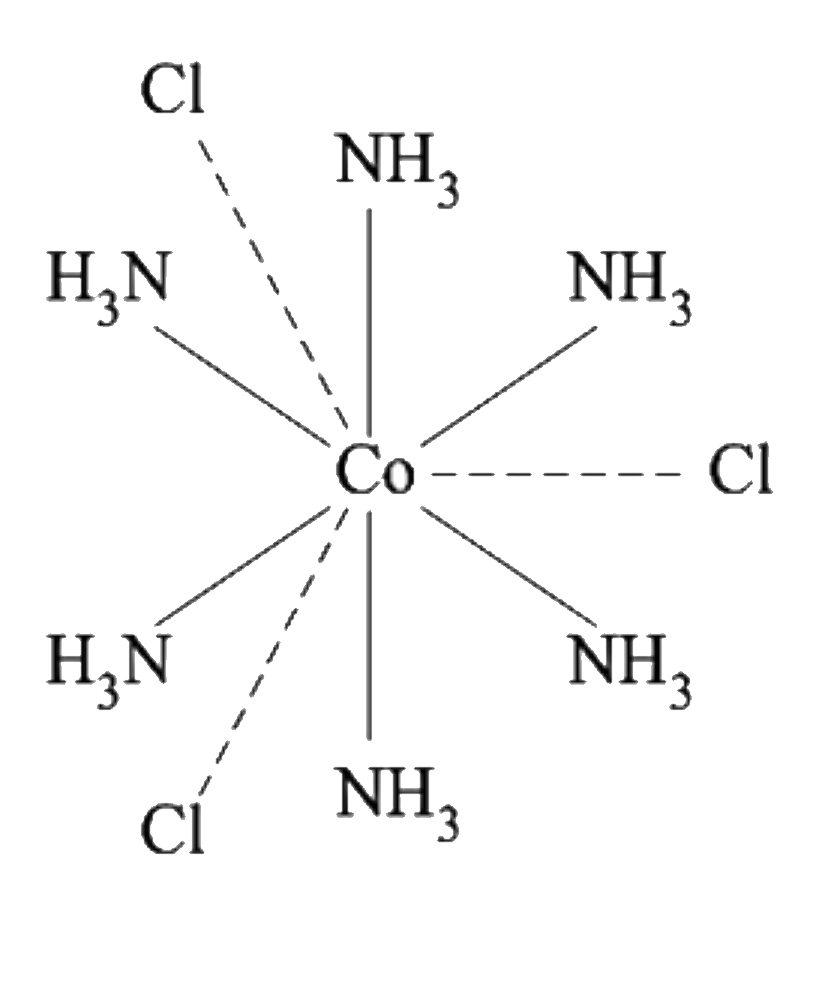
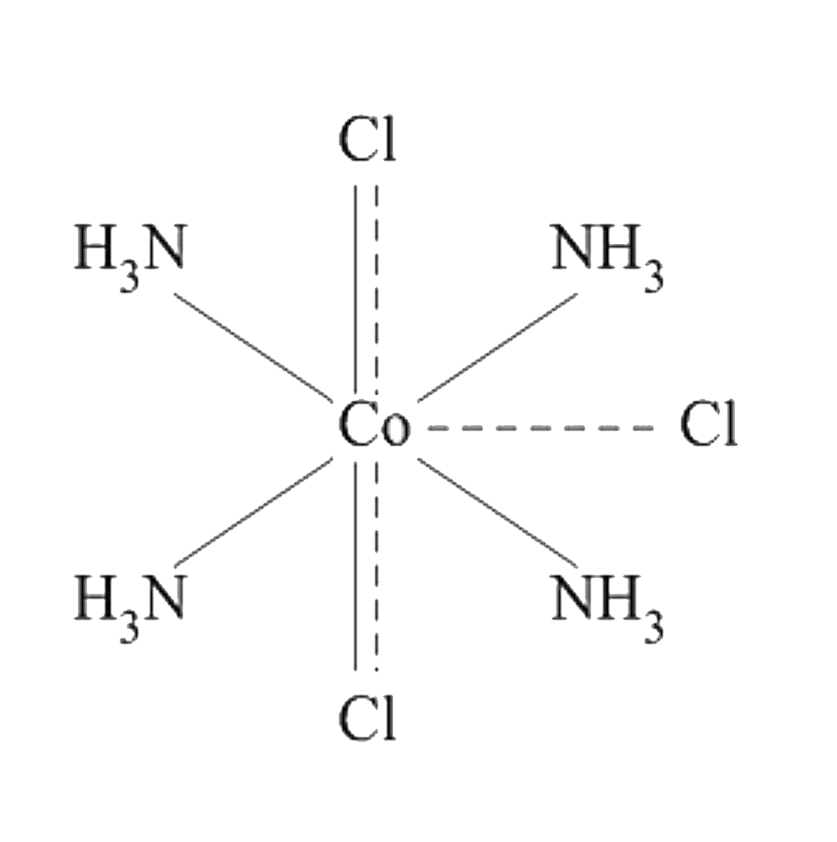
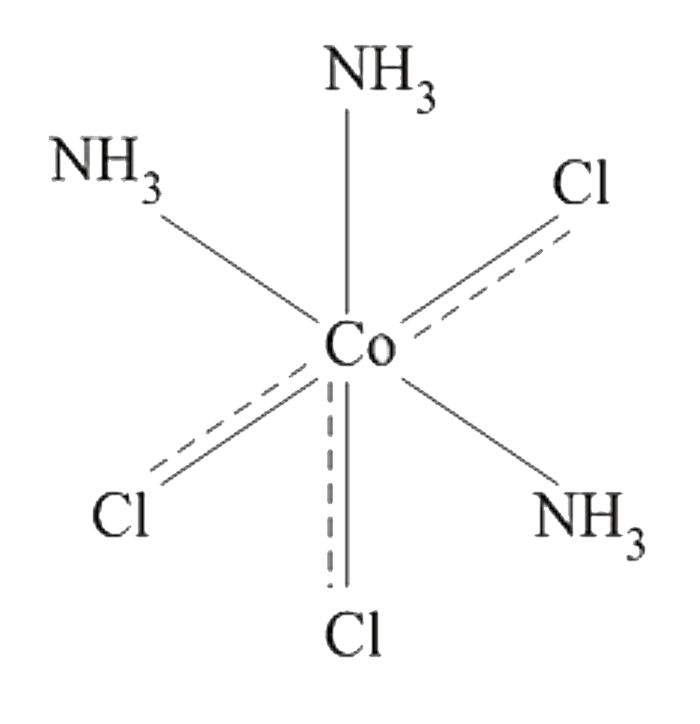
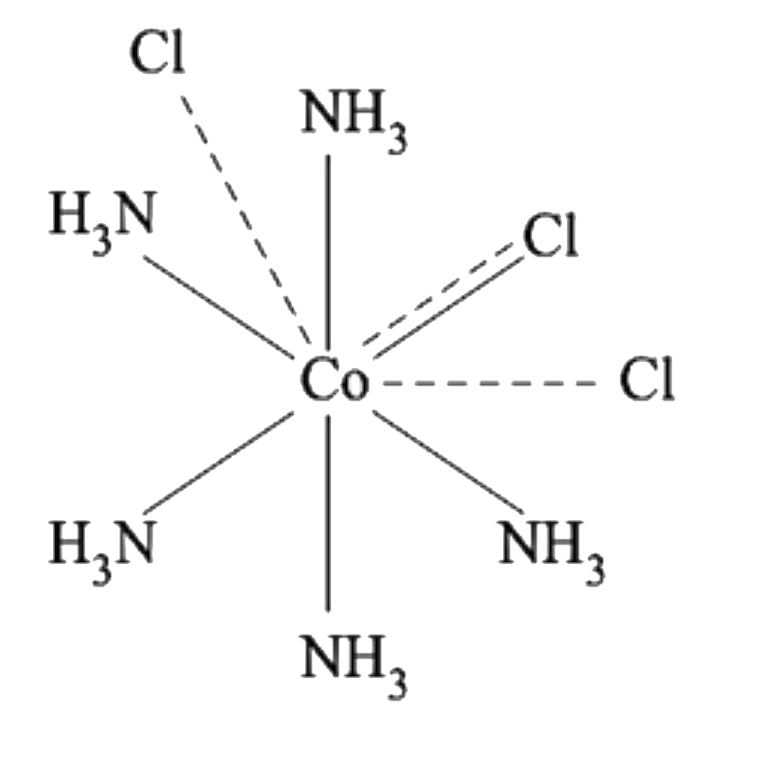
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The solution of which of the following will be non - conducting ?
Text Solution
|
- Which of the following when dissolving in water forms a solution which...
Text Solution
|
- Which of the following is non-conducting ?
Text Solution
|
- Which of the following is non-conducting ?
Text Solution
|
- The solution of which of the following will be non - conducting ?
Text Solution
|
- Which of the following is a non-aqueous solution ?
Text Solution
|
- Which of the following complexes is non-conducting electricity?
Text Solution
|
- উষ্ণতার ওপর নির্ভর করে নীচের কোন অক্সাইডটি তড়িৎ পরিবাহী বা অপরিবাহী হ...
Text Solution
|
- Which of the following compound form non conducting solution in water....
Text Solution
|