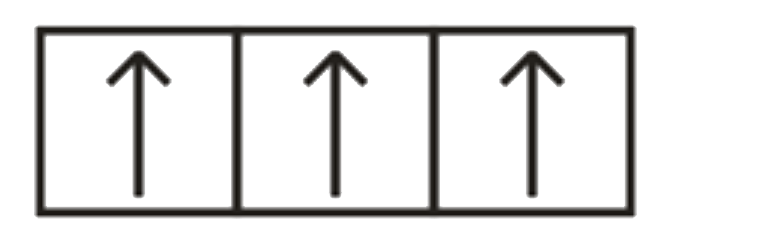
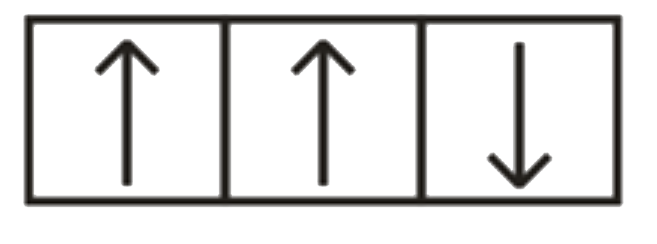
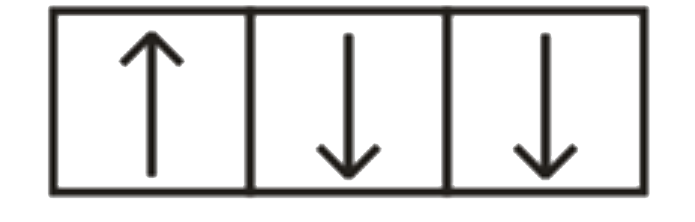
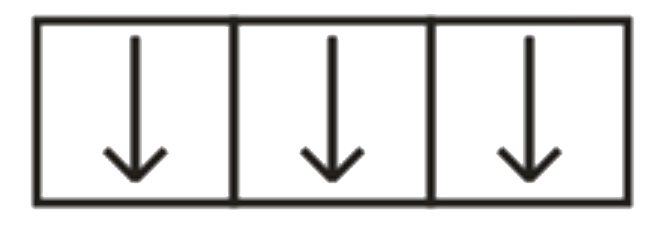
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following electronic configurations have spin multiplicit...
Text Solution
|
- Which of the following electronic configurations can leads to the form...
Text Solution
|
- Of the following element of which electronic configuration will have t...
Text Solution
|
- Which of the following electronic configurations has zero spin multipl...
Text Solution
|
- Spin angular momentum of an electron has no analogue in classical mech...
Text Solution
|
- Spin angular momentum of an electron has no analogue in classical mech...
Text Solution
|
- The valency shell electron configuration of an atom is 4s^(2)4p^(5). T...
Text Solution
|
- Sum of electronic spins of all electrons with the configuration 3d^(7)...
Text Solution
|
- Which of the following does not have abnormal electronic configuration...
Text Solution
|