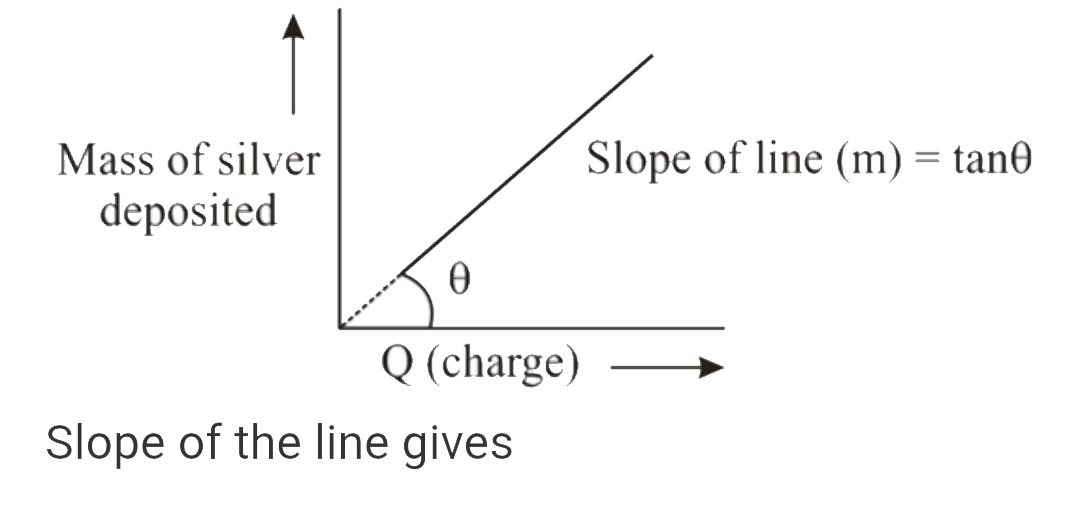
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In the electrolysis of silver nitrate, the mass of silver deposited is...
Text Solution
|
- If 0.5 amp current is passed through acidified silver nitrate soluti...
Text Solution
|
- The amount of electricity that can deposit 108g of silver from silver ...
Text Solution
|
- If 0.5 amp current is passed through acidified silver nitrate solution...
Text Solution
|
- Calculate the mass of silver deposited from silver nitrate solution by...
Text Solution
|
- 108 ग्राम सिल्वर को सिल्वर नाइट्रेट के विलयन से निक्षेपित करने के लिए ...
Text Solution
|
- In the electrolysis of silver nitrate, the mass of silver deposited is...
Text Solution
|
- Calculate the deposited mass of silver which is plotted against charge...
Text Solution
|
- If 0.5 amp current is passed through acidified silver nitrate solution...
Text Solution
|