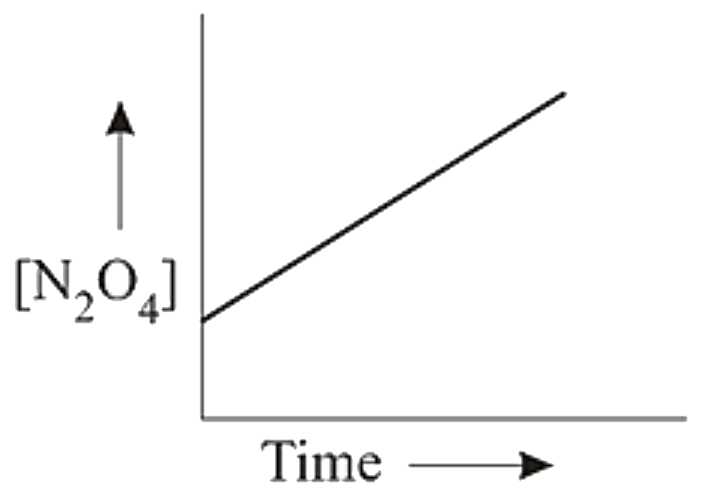
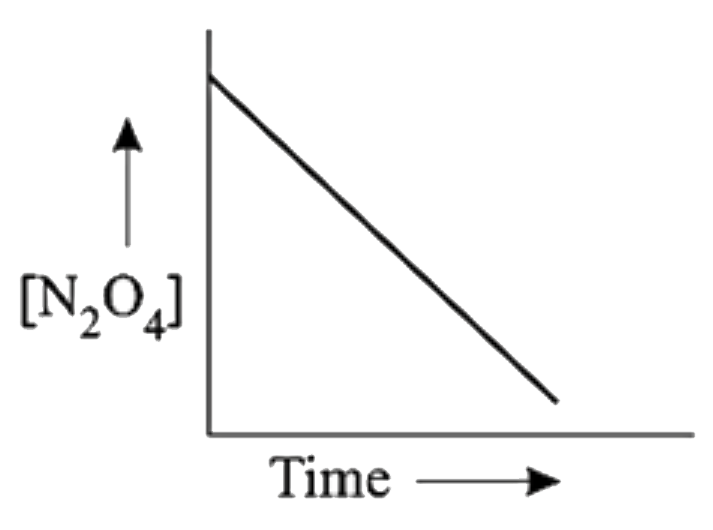
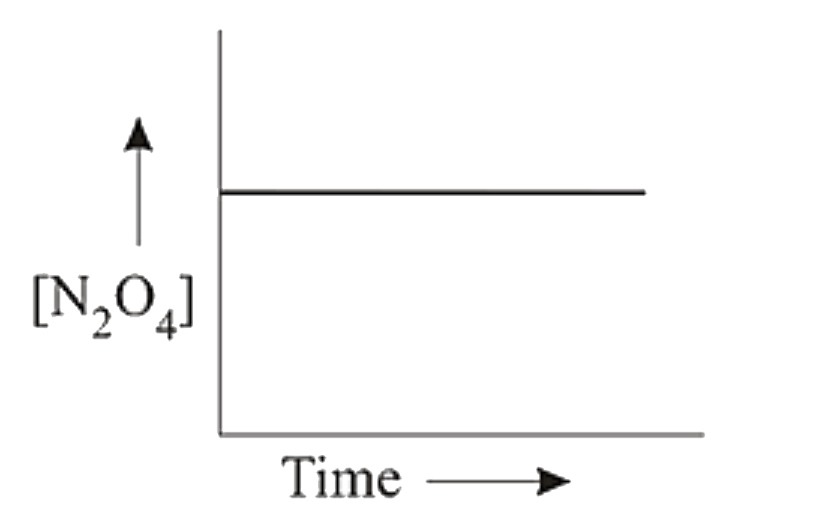
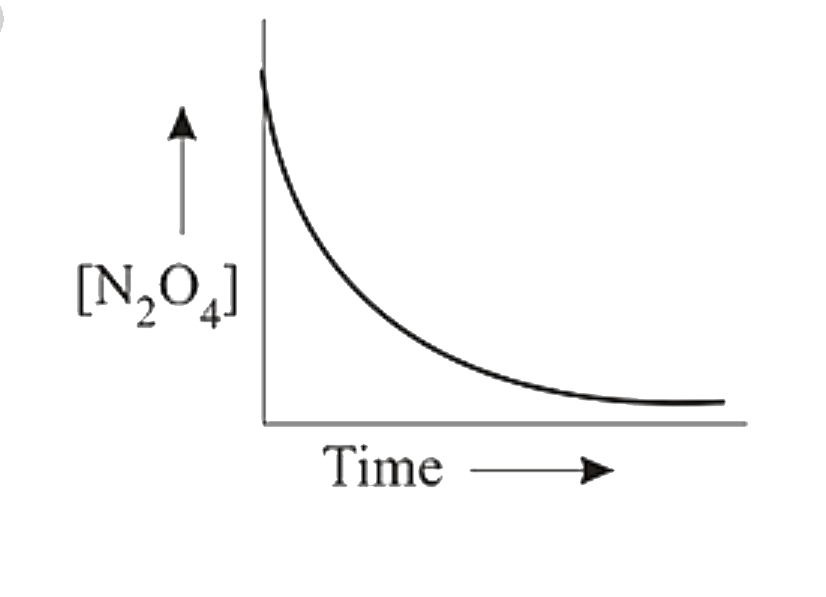
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Recommended Questions
- The reaction , N2O4(g)rarr2NO2(g) , is first order reaction , which of...
Text Solution
|
- For the reaction equilibrium , N2O4(g) hArr 2NO(2)(g) the concentratio...
Text Solution
|
- For the equilibrium N2O4(g)+heathArr2NO2(g), which of the following wi...
Text Solution
|
- N2O4(g) hArr 2NO2(g) . In this reaction, NO2 is 20% of the total volum...
Text Solution
|
- The reaction , N2O4(g)rarr2NO2(g) , is first order reaction , which of...
Text Solution
|
- For the following reaction at equilibrium 2NO2(g) harr N2O4(g) Delta...
Text Solution
|
- Kp for the reaction N2O4(g) leftrightarrow 2NO2(g) at 750K is 640torr....
Text Solution
|
- 2NO2(g) harr N2O4(g): DeltaH =-52.7 kJmol1 What happens when N2O4 is r...
Text Solution
|
- Kp for the reaction: N2O4(g) iff 2NO2(g) is 0.66 atm at 320 K. Calcula...
Text Solution
|