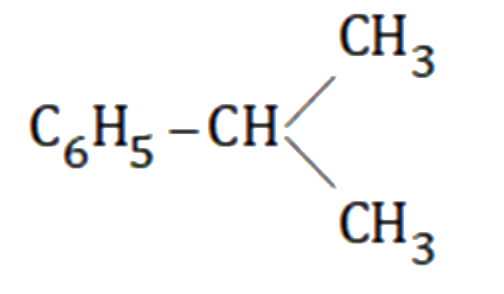A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The products of the reaction will be C6H6+CH3-CH2-CH2-Broverset("Anh...
Text Solution
|
- In the given reaction CH3-CH2-Broverset("Moist"Ag2O)rarr[X] [X] will b...
Text Solution
|
- In the given reaction CH3-underset(CH3)underset(|)overset(CH3)overset(...
Text Solution
|
- In the reaction sequence CH3-CH2-CH2-Broverset(AgCN) rarr (X) overset ...
Text Solution
|
- In the given reaction CH3-CH2-Broverset(Na//"dryether")rarr Product, p...
Text Solution
|
- The products of the reaction will be C6H6+CH3-CH2-CH2-Broverset("Anh...
Text Solution
|
- In the given reaction CH3-CH2-underset(CH3)underset(|)(CH)-CH2-Brovers...
Text Solution
|
- Predict the product of the reaction CH3-CH2-CH2 - O-CH3 + HBr to
Text Solution
|
- Predict the products of the following reactions CH3-CH2-CH2-O-CH3+HBr ...
Text Solution
|
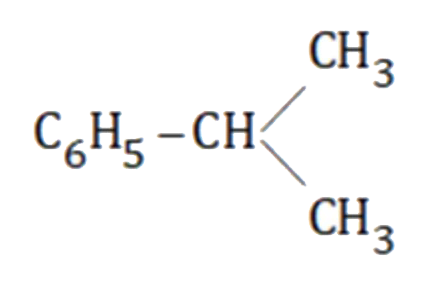
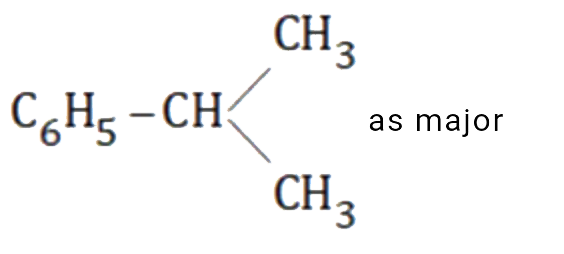 as major product and `C_6H_5-CH_2-CH_2-CH_3` as minor product.
as major product and `C_6H_5-CH_2-CH_2-CH_3` as minor product.