A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
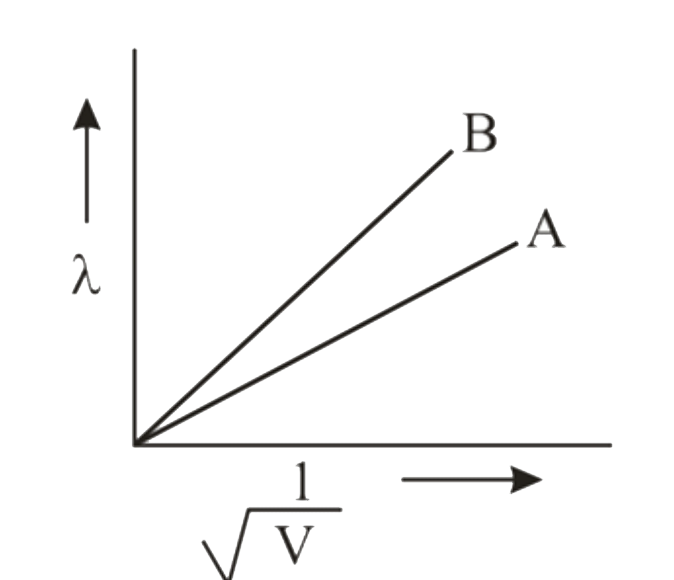
- de Broglie wavelengths of two particles A and B are plotted against (1...
Text Solution
|
- The two lines A and B in fig. shoe the phot of de Broglie wavelength (...
Text Solution
|
- Plot a graph showing variation of de-broglie wavelength lambda versus ...
Text Solution
|
- For two particles A and B, curves are plotted sqrtV against de-Broglie...
Text Solution
|
- de Broglie wavelengths of two particles A and B are plotted against (1...
Text Solution
|
- Plot a graph showing variation of a de Broglie wavelength (lamda) asso...
Text Solution
|
- द्रव्यमान m तथा आवेश q के एक कण को V विभव द्वारा त्वरित किया जाता है। ...
Text Solution
|
- Plot a graph showing variation of de-Broglie wavelength lambda versus ...
Text Solution
|
- Plot a graph showing variation of de-Broglie wavelength lamda versus 1...
Text Solution
|