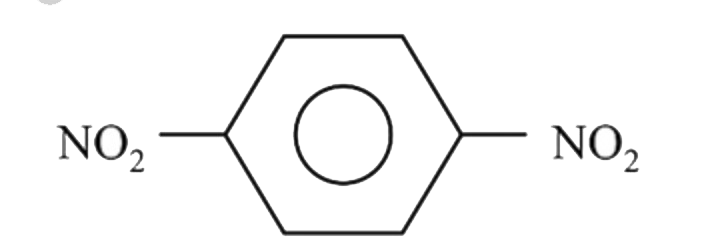
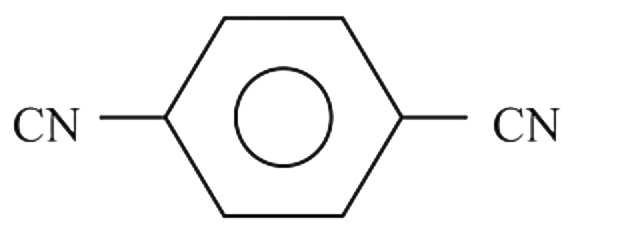
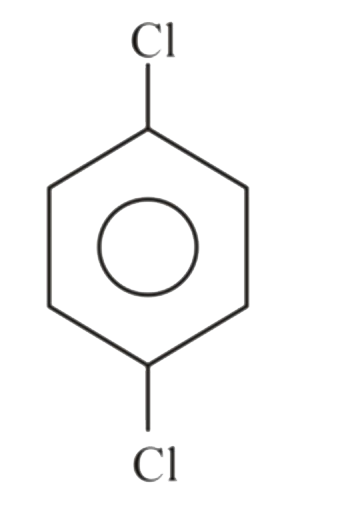
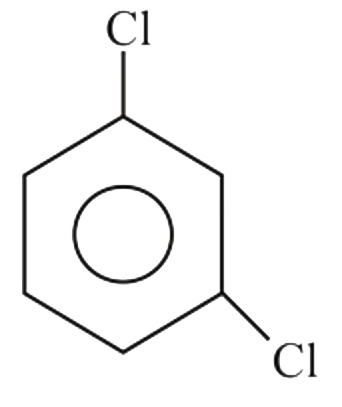
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following compounds would you expect to have a dipole mom...
Text Solution
|
- Of the following compounds, which will have a zero dipole moment ?
Text Solution
|
- Which of the following compounds have zero dipole moment ?
Text Solution
|
- Which of the following compounds have finite dipole moments ?
Text Solution
|
- Which of the following would have a permanent dipole moment ?
Text Solution
|
- Which of the following compounds will have the highest dipole moment ?
Text Solution
|
- Which of the following compounds would you expect to have a dipole mom...
Text Solution
|
- Of the following compounds, which will have zero dipole moment ?
Text Solution
|
- निम्न यौगिकों में से किसका द्विध्रुप आघूर्ण ( dipole moment ) सबसे अधि...
Text Solution
|