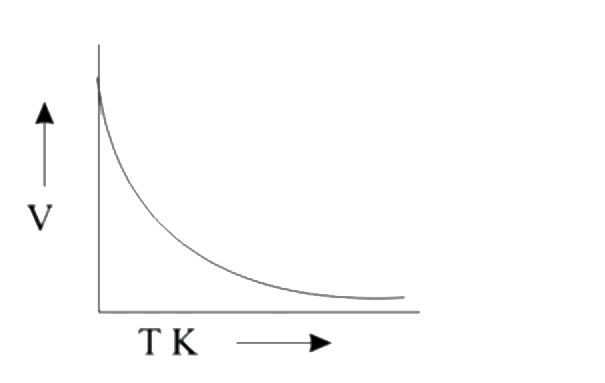
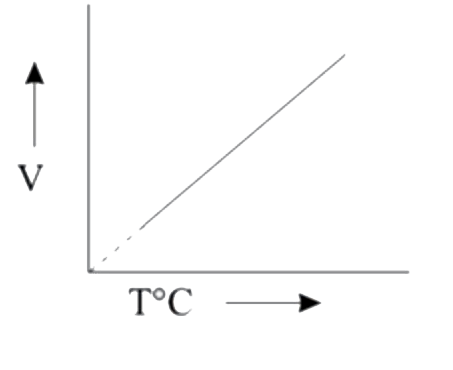
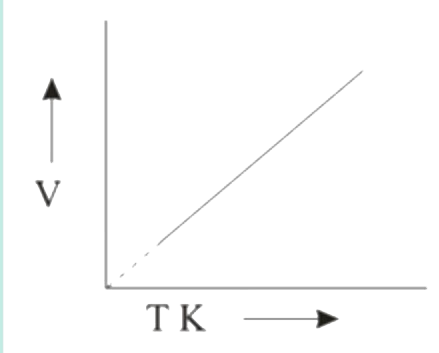
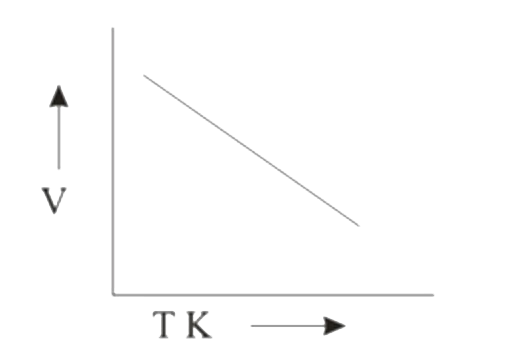
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following is a correct plot of the volume of fixed amount...
Text Solution
|
- Assertion: The product of pressure and volume for a fixed amount of ga...
Text Solution
|
- The slope of 'P' v/s "T" plot at constant volume for a fixed amount of...
Text Solution
|
- A plot of volume (V) versus temperature (T) for a gas at constant pres...
Text Solution
|
- Show that Volume of a fixed amount of an ideal gas is a state func...
Text Solution
|
- For fixed mass of an ideal gas a constant temperature. Which is correc...
Text Solution
|
- Plot density vs pressure for a fixed mass of an ideal gas at a constan...
Text Solution
|
- Which of the following curve is correct for a given amount of an ideal...
Text Solution
|
- Which of the following is a correct plot of the volume of fixed amount...
Text Solution
|