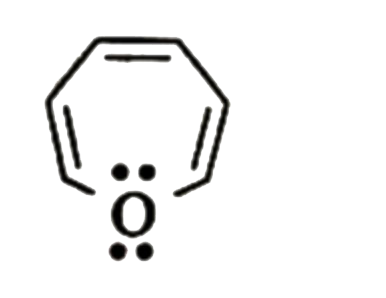
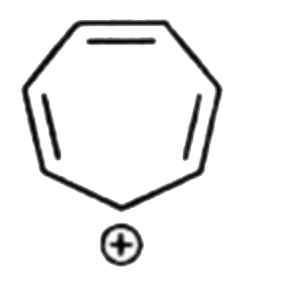
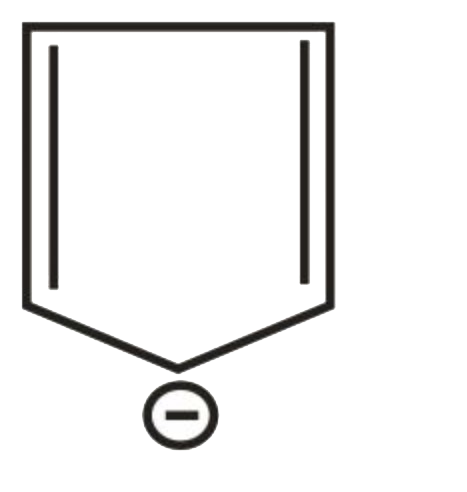
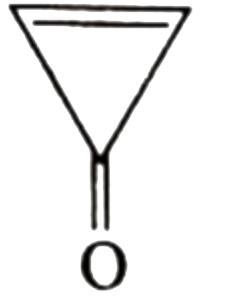
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which one of the following compound is not a planar ?
Text Solution
|
- Which of the following compound(s) is /are planar about nitrogen?
Text Solution
|
- Which of the following compounds have planar molecular configuration?
Text Solution
|
- Which one of the following is planar ?
Text Solution
|
- Among the following compound which one is planar in shape
Text Solution
|
- Which one of the following is planar?
Text Solution
|
- Which of the following compound is planar and non-polar ?
Text Solution
|
- Which of the following compound is planar and non-polar?
Text Solution
|
- Which of the following compound or ion is planar?
Text Solution
|