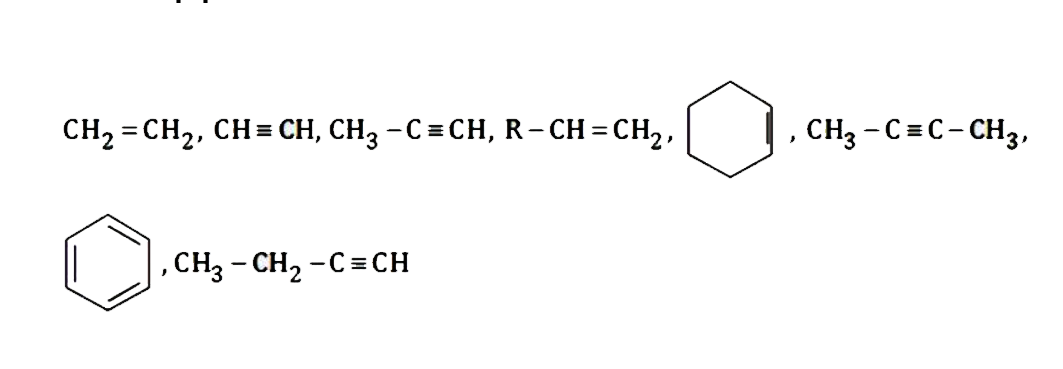Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- How many of these compounds can react with ammoniacal silver nitrate s...
Text Solution
|
- Which of the following will not react with an ammoniacal silver nitrat...
Text Solution
|
- Which of the following will not give white precipitate with ammoniacal...
Text Solution
|
- Which of the followings compounds will gives white ppt. with ammonical...
Text Solution
|
- Which of the following will not give white precipitate with ammoniacal...
Text Solution
|
- Ammoniacal silver nitrate forms a white precipitate easily with:
Text Solution
|
- Assertion. But 1-yne and but-2-yne can be distinguished by ammoniacal ...
Text Solution
|
- The simplest hydrocarbon which reacts with ammoniacal silver nitrate t...
Text Solution
|
- How many of these compounds can react with ammonical silver nitrate so...
Text Solution
|