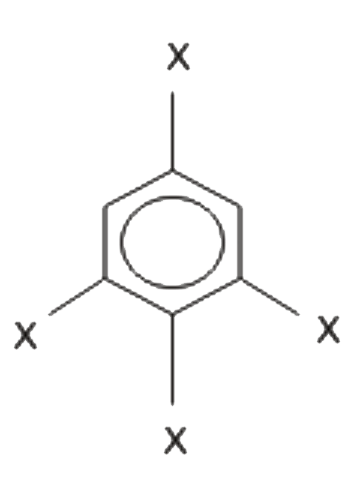
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The dipole moment of is 1.5 D. The dipole Moment of in Debye is
Text Solution
|
- If the molecule of HCl were totally polar, the expected value of dipol...
Text Solution
|
- A polar covalent bond with positive and negative charge centres at its...
Text Solution
|
- A polar covalent bond with positive and negative charge centres at its...
Text Solution
|
- A polar covalent bond with positive and negative charge centres at its...
Text Solution
|
- The dipole moment of is 1.5D what will be the dipole moment of
Text Solution
|
- The dipole moment of chlorobenzene is 1.73 D . The dipole moment of p-...
Text Solution
|
- Debye , the unit of dipole moment is related to the SI unit of dipole ...
Text Solution
|
- Polar covalent molecules exhibit dipole moment. Dipole moment is equal...
Text Solution
|
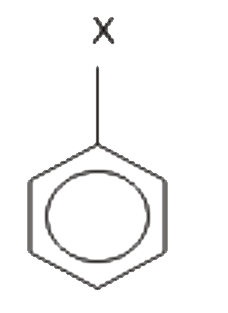 is 1.5 D.
is 1.5 D.