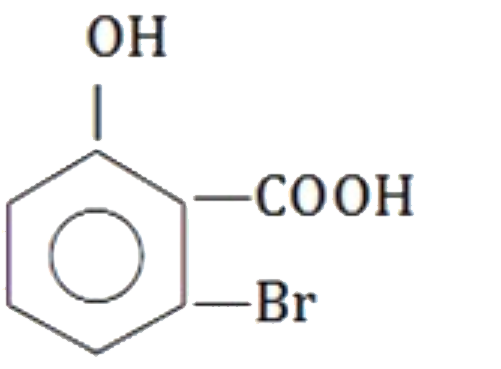
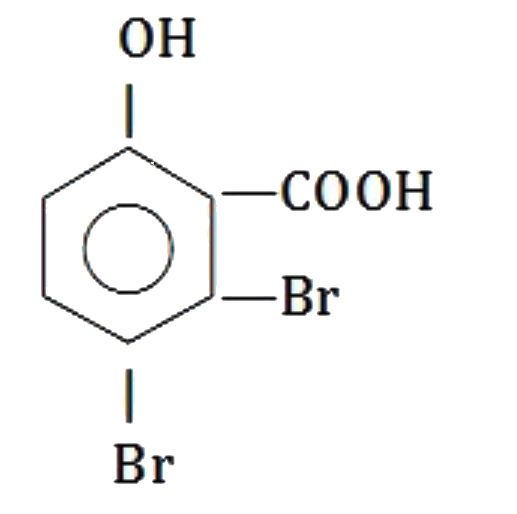
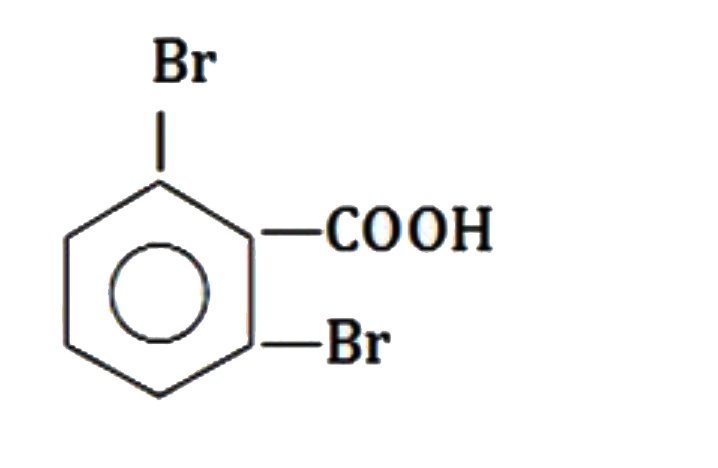
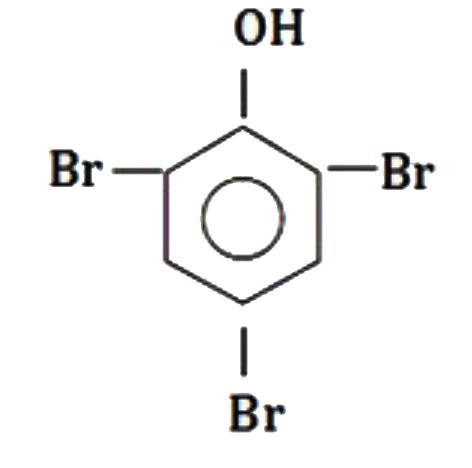
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The reaction of p-HOC(6)H(4)COOH with excess Br(2) forms
Text Solution
|
- Consider the acidity of the carboxylic acids: (1) PhCOOH (2) o-NO(2) C...
Text Solution
|
- An unknown compound (A) has a molecular formula C(4)H(6). When (A) is ...
Text Solution
|
- Arrange in order of decreasing reactivity in electrophilic addition: ...
Text Solution
|
- Consider the acidity of the carboxylic acids : (a) PhCOOH (b) o-NO(2)C...
Text Solution
|
- Consider the acidity of the carboxylic acids : (i) PHCOOH" "(ii) o-NO(...
Text Solution
|
- Consider the acidity of the carboxylic acids: (1) PhCOOH (2) o-NO(...
Text Solution
|
- The reaction of p-HOC(6)H(4)COOH with excess Br(2) forms
Text Solution
|
- 25 ml of a dilute aqueous solution of p-hydroxy benzoic acid is titrat...
Text Solution
|