A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
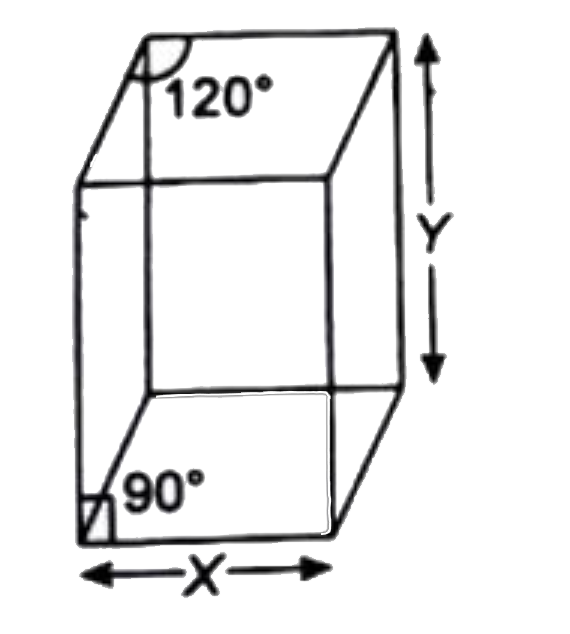
- X=5Å Y=8Å Molar mass of solid ="259.8 g mol"^(-1) A solid crysta...
Text Solution
|
- A solid crystallines in a hexagonal structures as shown in the figure....
Text Solution
|
- A(2)B molecules (" molar mass " = 259.8 g//"ml") crystallises in a hex...
Text Solution
|
- Ice crystallises in a hexagonal lattice having a volume of the unit ce...
Text Solution
|
- A metal with a molar mass of 75g*mol^(-1) crystallises in a cubic latt...
Text Solution
|
- An element has unit cell made up of planes as shown below: Co-ordinati...
Text Solution
|
- X=5Å Y=8Å Molar mass of solid ="259.8 g mol"^(-1) A solid crysta...
Text Solution
|
- "Molar mass of solid = 259.8 g mol"(-1) A solid crystallises in hexago...
Text Solution
|
- A molecule A(2)B(MW = 166.4) occupies triclinic lattice with a = 5Å, b...
Text Solution
|