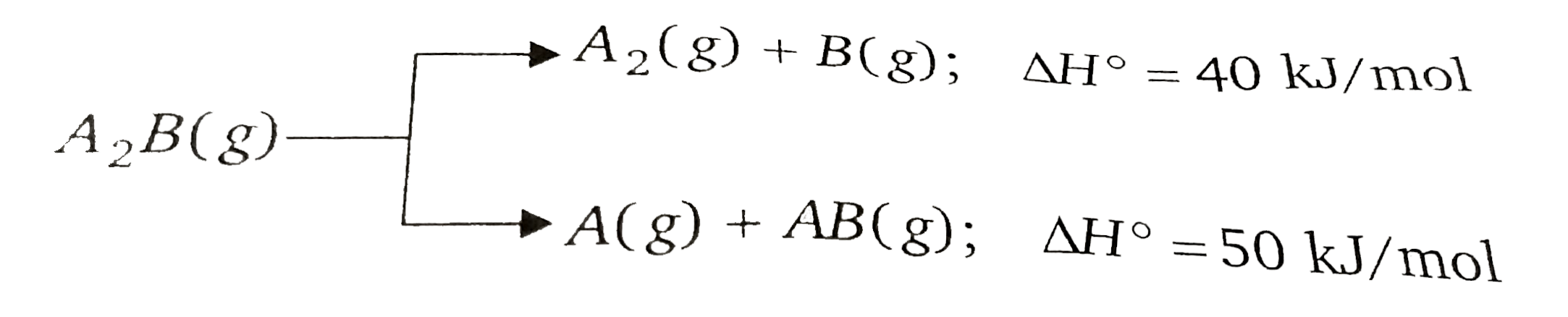A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Substance A(2)B(g) can undergoes decomposition to form two set of prod...
Text Solution
|
- In the reaction A(2)(g) + 4B(2)(g)hArr2AB(4)(g) , Delta H lt 0 . The d...
Text Solution
|
- In the following reaction started only with A(B), 2A(B)(g) hArr3A(2)(g...
Text Solution
|
- Substance A(2)B(g) can undergo decomposition to form of set of product...
Text Solution
|
- Ethanol can undergo decompostion to form two sets of products. If the ...
Text Solution
|
- Substance A(2)B(g) can undergoes decomposition to form two set of prod...
Text Solution
|
- A(2)B((g)) is introduced in a vessel at 1000K . If partial pressure of...
Text Solution
|
- In a closed vessel, 5 moles of A(2)(g) and 7 moles of B(2) (g) are rea...
Text Solution
|
- यदि S={a,b,c,d,e,f,g}, तो ज्ञात करो की निम्नलिखित समुच्यो S से विभाग स...
Text Solution
|