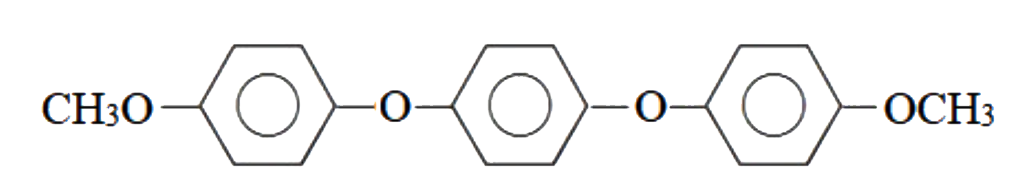
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- How many moles of HI consumed in above reaction?
Text Solution
|
- How may molecules of RMgX are consumed in the above reaction.
Text Solution
|
- Number of moles (x) of Grignard reagent consumed in the above reaction...
Text Solution
|
- How many mole of NaOH are consumed in Hoffmann Bromamide reaction?
Text Solution
|
- How many mole of 'HI' will react with
Text Solution
|
- How many molecules of RMgX are consumed in the above given reaction ?
Text Solution
|
- How many moles of Grignard's reagent will be consumed per mole of foll...
Text Solution
|
- How many molecules of RMgX are consumed in the above given reaction?
Text Solution
|
- How many moles of HI consumed in above reaction?
Text Solution
|