A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- For a second order reaction, 2Ararr Products, a plot of log t(1//2) v...
Text Solution
|
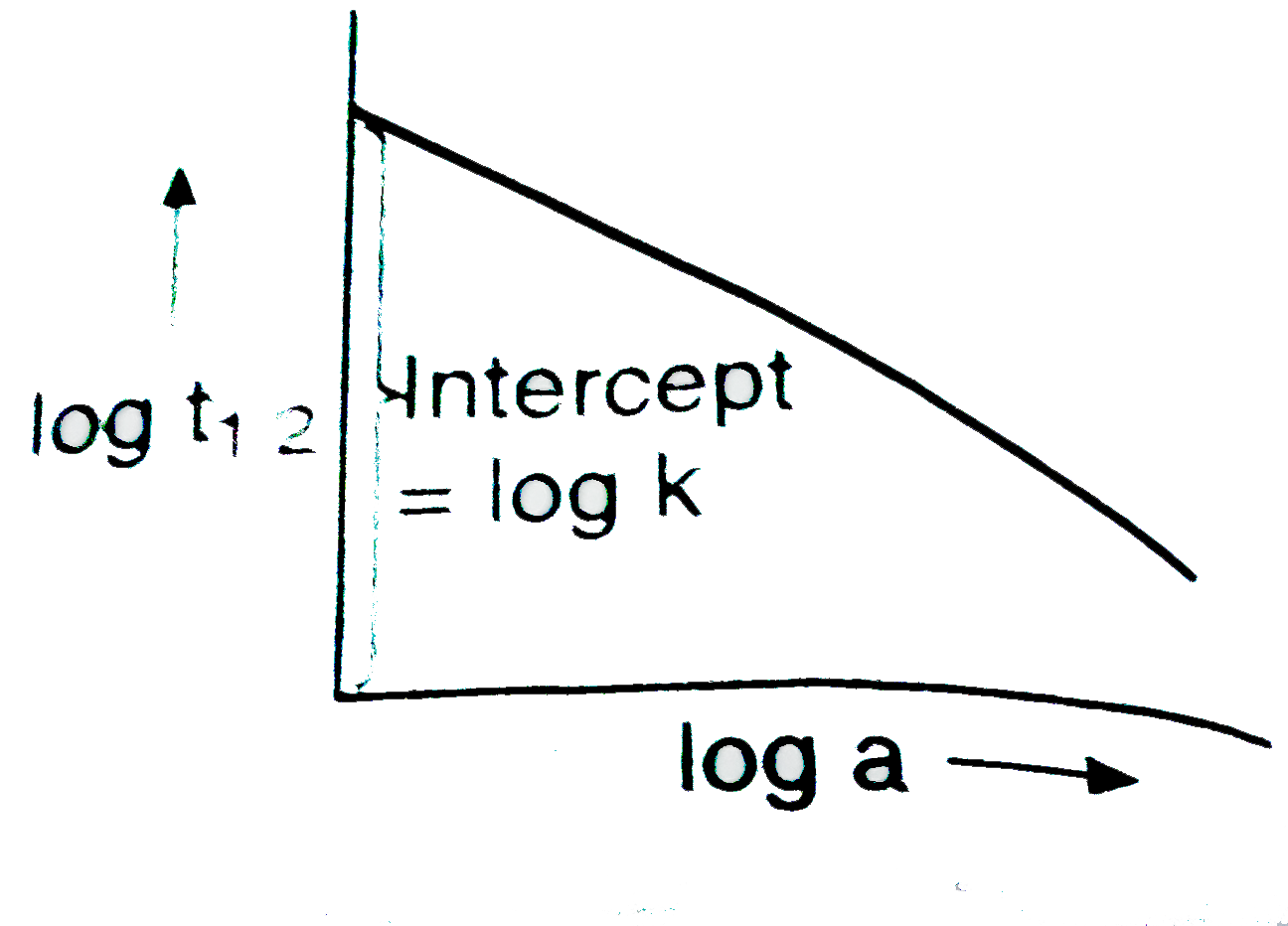
- Following is the graph between log t(1//2) and log a (a initial concen...
Text Solution
|
- For a first order reaction, the reaction, the plot of log C against 't...
Text Solution
|
- What will be the order of reaction for a chemical change having log t(...
Text Solution
|
- What will be the order of reaction and rate constant for a chemical ch...
Text Solution
|
- Following is the graph between log T(50) and log a (a = initial concen...
Text Solution
|
- For a second order reaction, 2Ararr Products, a plot of log t(1//2) vs...
Text Solution
|
- For a second order reaction, 2" A"to Products, a plot of log t(1//2) v...
Text Solution
|
- Plotting log(10)t(1//2) against log (10)[A0] (where A0 is the initial...
Text Solution
|