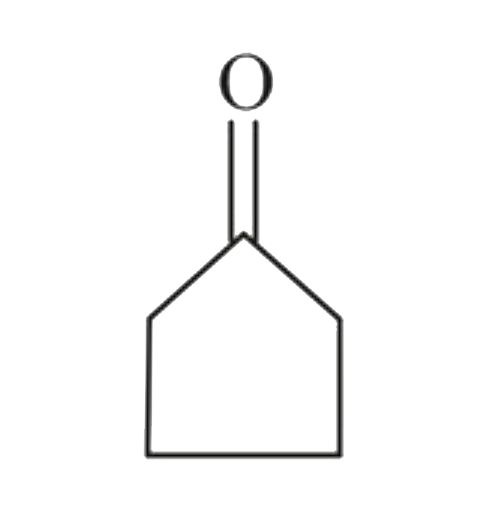
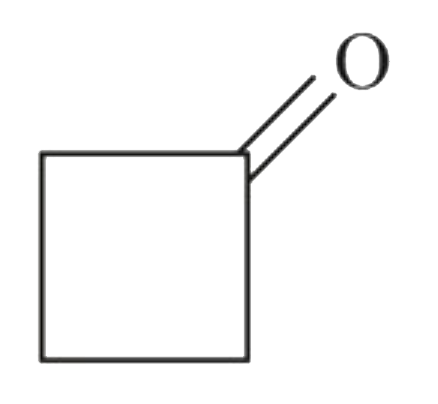
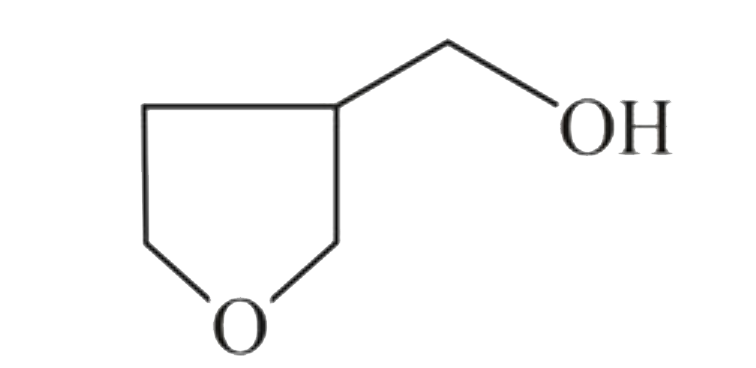
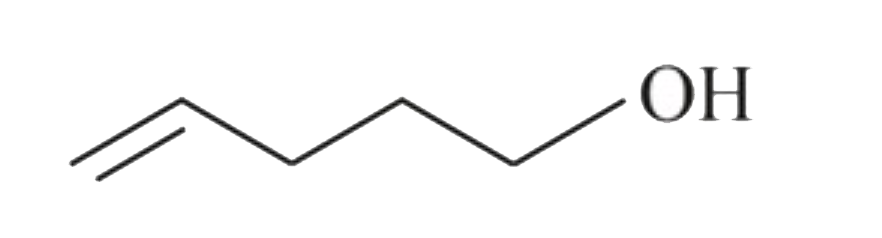
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- (A)(C(4)H(8)O)overset(H(3)O^(o+))rarr(B)overset(CrO(3))underset("aceti...
Text Solution
|
- Complete the following reaction : (a) (b) ( c) (d) ( e) ...
Text Solution
|
- H(2)O^(18) + Na rarr underset("(base)")((A))+(B) CH(3)-overset(O)ove...
Text Solution
|
- CH(3)-(CH(2))(3)-OH overset(CH(3)-underset(O)underset(||)overset(O)ove...
Text Solution
|
- C(6) H(5) (CH(2))(5) overset(O)overset(||)(C) - Cl underset(CS(2))over...
Text Solution
|
- CH(3)-underset(Br)underset(|)CH-CH(2)-overset(O)overset(||)C-CH(3)unde...
Text Solution
|
- 'A' +NaOH rarr 'B' ("gas") +'C' overset(Zn)rarr 'B' underset(("gas...
Text Solution
|
- CH(3)CH(2)CHOunderset(H(2)O)overset(CH(3)MgBr)rarr[A]underset("Heat")o...
Text Solution
|
- (i) CHequivCHoverset(H(2)O)underset(H(2)SO(4)//HgSO(4))rarr[A]overset(...
Text Solution
|