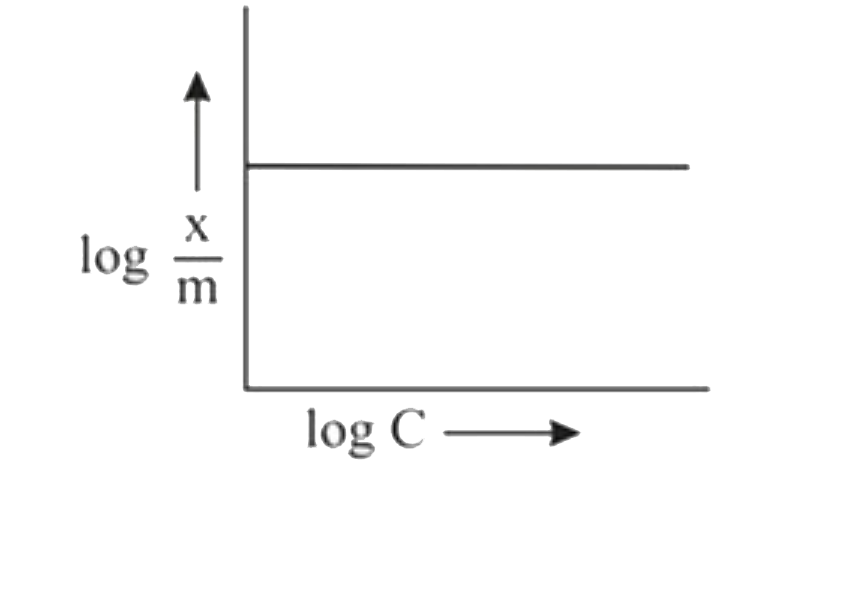
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The degree of adsorption of solution on solid surface depends on conce...
Text Solution
|
- किसी विलयन में विलेय की सांद्रता तथा उसमें उपस्थित ठोस अधिशोषक के इकाई...
Text Solution
|
- The degree of adsorption of solution on solid surface depends on conce...
Text Solution
|
- (x)/(m)=KC^(1//n) where C = Concentration of a m solution, x = wt of a...
Text Solution
|
- The following are some statements about adsorption of solutes from the...
Text Solution
|
- (x)/(m)=KC^(1//n) where C = Concentration of a m solution, x = wt of a...
Text Solution
|
- The following are some statements about adsorption of solutes from the...
Text Solution
|
- For the adsorption of solution on a solid surface x/m = kc^(1//n) . Ad...
Text Solution
|
- the adsorption of solids, from a solution is called
Text Solution
|