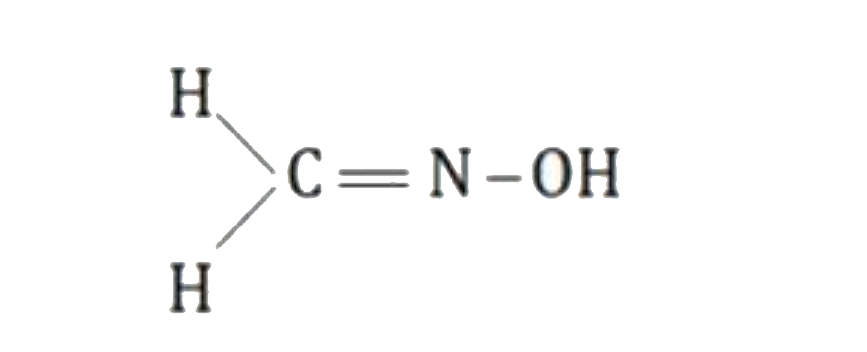A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which one of the following will not give alkyl cyanide on treatment wi...
Text Solution
|
- P(2)O(5) is heated with water to give
Text Solution
|
- Amide on heating with P(2)O(5) gives
Text Solution
|
- (A) Treatment of alkyl halide with alcoholic solution of potassium cya...
Text Solution
|
- P(2)O(5) is heated with water to give
Text Solution
|
- ऐमाइड्स को P(2)O(5) के साथ गर्म करके शुद्ध सायनाइड्स को प्राप्त किया ज...
Text Solution
|
- Alkyl cyanides upon hydrolysis give :
Text Solution
|
- Which one of the following will not give alkyl cyanide on treatment wi...
Text Solution
|
- Acetaldoxime reacts with P(2)O(5) to give :
Text Solution
|