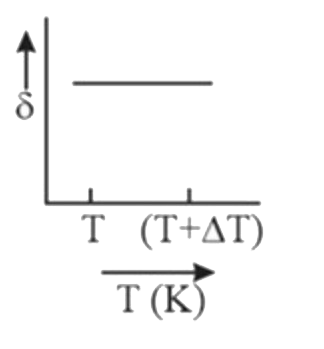
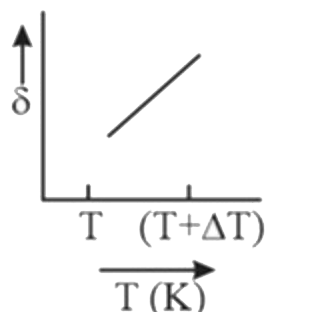
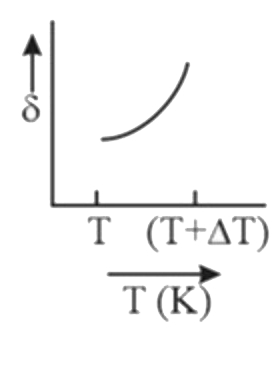
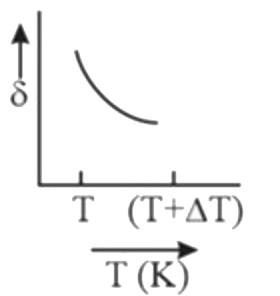A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- An ideal gas is initially at temperature T and volume V. ITS volume is...
Text Solution
|
- An ideal gas is initially at temperature T and volume V. Its volume is...
Text Solution
|
- यदि नियत आयतन पर 1 मूल गैस का ताप DeltaT बढ़ाने से उसकी आन्...
Text Solution
|
- An ideal gas is initially at temperature T and volume V . Its volume i...
Text Solution
|
- An ideal gas is initially at temperature T and volume V. Its volume is...
Text Solution
|
- An ideal gas is initiaily at temperature T and volumoeV. lts volume is...
Text Solution
|
- An ideal gas is initially at temperature T and volume V. Its volume is...
Text Solution
|
- An ideal gas is initially at temperature T and volume V. Its volume is...
Text Solution
|
- An ideal gas is initially at temperature T and volume V. ITS volume is...
Text Solution
|