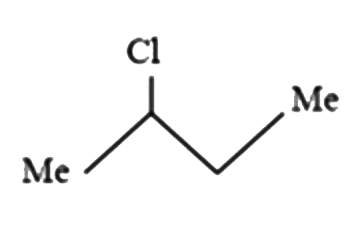
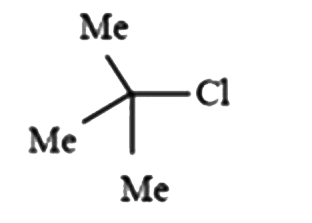
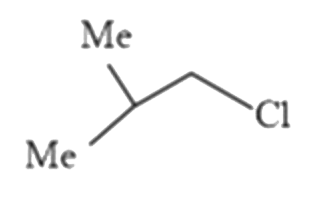
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Equal amount of an RCl(C4 H9Cl) is reacted at the same temperature wit...
Text Solution
|
- Equal amount of an RCl(C4 H9Cl) is reacted at the same temperature wit...
Text Solution
|
- In two separate experiments equal quantities of alkyl halide, C4H9Cl, ...
Text Solution
|
- Equal volumes of M NaOH and 0.3 M KOH are mixed in an experiment. Fin...
Text Solution
|
- Equal amount of an RCl(C4 H9Cl) is reacted at the same temperature wit...
Text Solution
|
- Arrange the following in the increasing order of boiling point: C2 ...
Text Solution
|
- Arrange the following compounds in the increasing order of boiling poi...
Text Solution
|
- In two separate experiments equal quantities of alkyl halide, C4H9Cl ,...
Text Solution
|
- The resistance of two electrolytes X and Y ere found to be 45 and 100 ...
Text Solution
|