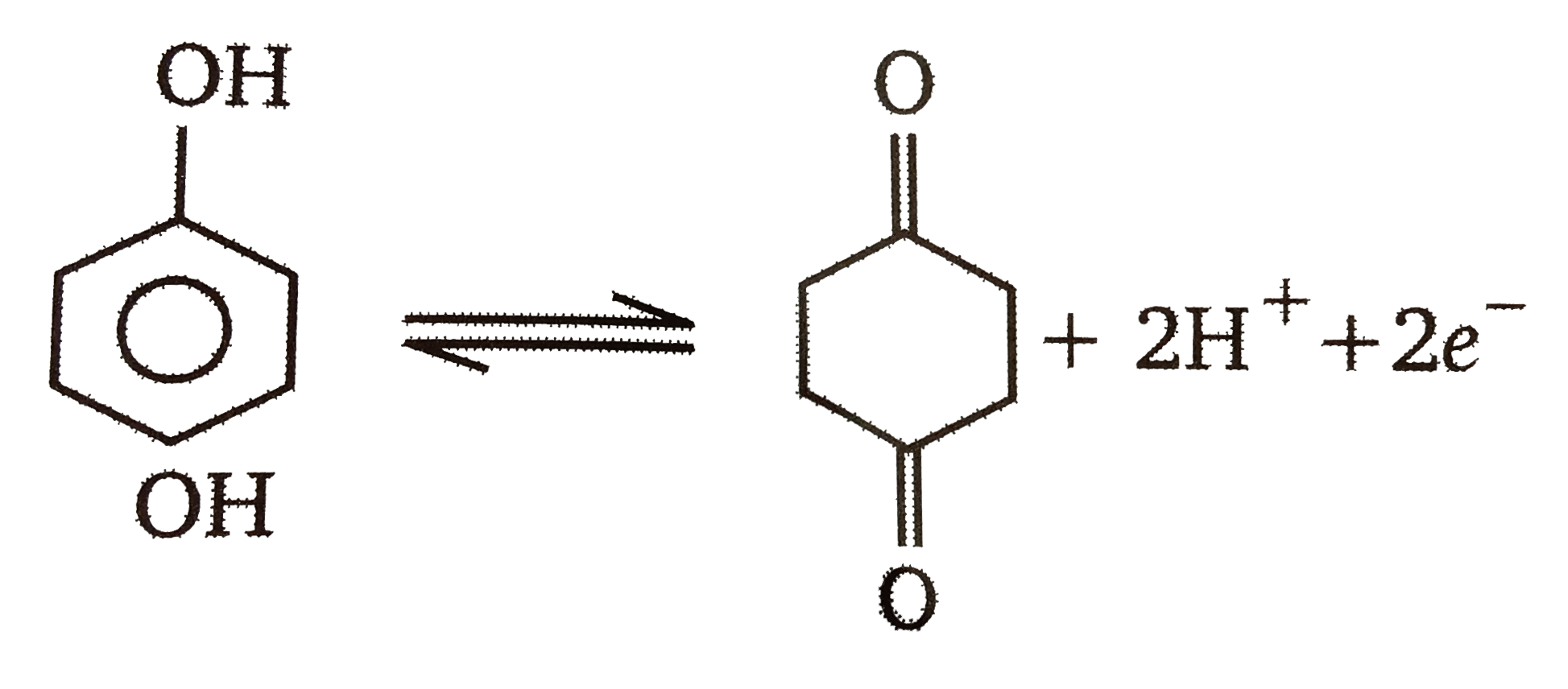
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- At pH = 2, E(("Quinhydrone"))^(@) = 1.30 V, E("Quinhydrone") will be :
Text Solution
|
- The quinhydrone electrode (Q,H^(o+)|H^(2)Q) is used in conjunction wit...
Text Solution
|
- The satandard EMF of quinhydrone is 0.699V . The EMF of the quinhydron...
Text Solution
|
- The calomel and quinhydrone electrodes are reversible with respect to ...
Text Solution
|
- At pH = 2, E(("Quinhydrone"))^(@) = 1.30 V, E("Quinhydrone") will be :
Text Solution
|
- Quinhydrone Electrode
Text Solution
|
- The term quinhydrone means
Text Solution
|
- At pH = 2, E(("Quinhydrone"))^(@) = 1.30 V, E("Quinhydrone") will be :
Text Solution
|
- For the half cell, +2H^(+)2e^(-).E^(0)= 1.30 V. At pH = 2, elec...
Text Solution
|