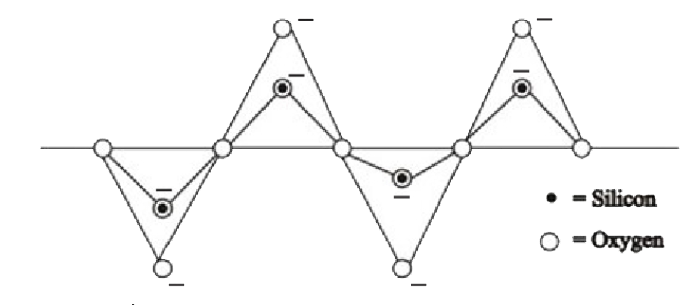
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Pyroxenes are class of silicate minerals, which exhibit a polymeric ch...
Text Solution
|
- Which of the following anions is present in the chain structure of sil...
Text Solution
|
- The minerals having silicate chains are collectively called
Text Solution
|
- A silicate anion in a mineral is a linear chain of three silicate tetr...
Text Solution
|
- Which of the following anions is present in the chain structure of sil...
Text Solution
|
- beta-D- ग्लूकोज इकाई की बहुलक संरचना कौन-सा पॉलिसेकेराइड प्रदर्शित करत...
Text Solution
|
- Which of the following is present in the chain structure of silicate-
Text Solution
|
- Pyroxenes are class of silicate minerals, which exhibit a polymeric ch...
Text Solution
|
- सिलिकेटों की शृंखला संरचना में निम्न ऋणायनों में कौन-सा उपस्थित होता ह...
Text Solution
|