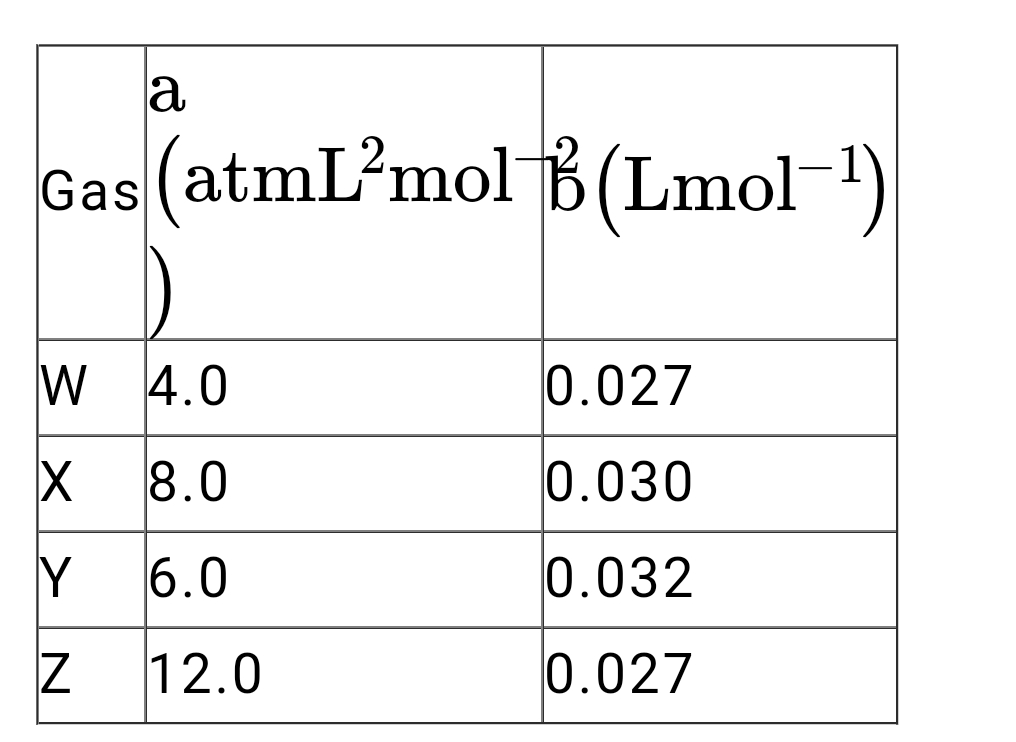
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The following are the Vander Waal's parameters for four gases W, X, Y ...
Text Solution
|
- Which one of these gases has the highest critical temperature ? .
Text Solution
|
- Which one of the following gases has the highest critical temperature ...
Text Solution
|
- The Van der Waal's parameters for gases W,X,Y and Z are- Which on...
Text Solution
|
- Which one of the following gases has the highest critical temperature ...
Text Solution
|
- निम्नलिखित गैसों में से किसका क्रांतिक ताप उच्चतम है ?
Text Solution
|
- The van dar Waals parameters for gases W, X, Y and Z are {:(Gas,a(at...
Text Solution
|
- The Van der Waal's parameters for gases W,X,Y and Z are- Which one of ...
Text Solution
|
- The following are the Vander Waal's parameters for four gases W, X, Y ...
Text Solution
|