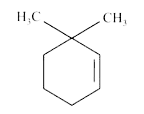
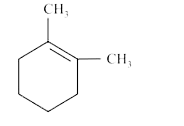
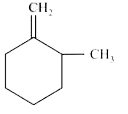
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- 1 - Bromo -2, 2 - dimethylcyclohexane on treatment with methanol give...
Text Solution
|
- Trans-1-2-dimethylcyclohexane
Text Solution
|
- Which one of the following will give acetaldehyde and methanol on trea...
Text Solution
|
- Which of the following methods give rise to 1-bromo-2- phenylethane?
Text Solution
|
- S(N)^(2) प्रतिस्थापन के प्रति अभिक्रियाशीलता के आधार पर इन यौगिकों के ...
Text Solution
|
- निम्न यौगिकों को प्रतिस्थापन हेतु क्रियाशीलता के कर्म में व्यवस्थित की...
Text Solution
|
- Write the structure of the compound 1-Bromo-4-sec-butyl-2-methylbenzen...
Text Solution
|
- Among the following compounds how many has a stereoisomer that is a me...
Text Solution
|
- 1 - Bromo -2, 2 - dimethylcyclohexane on treatment with methanol give...
Text Solution
|