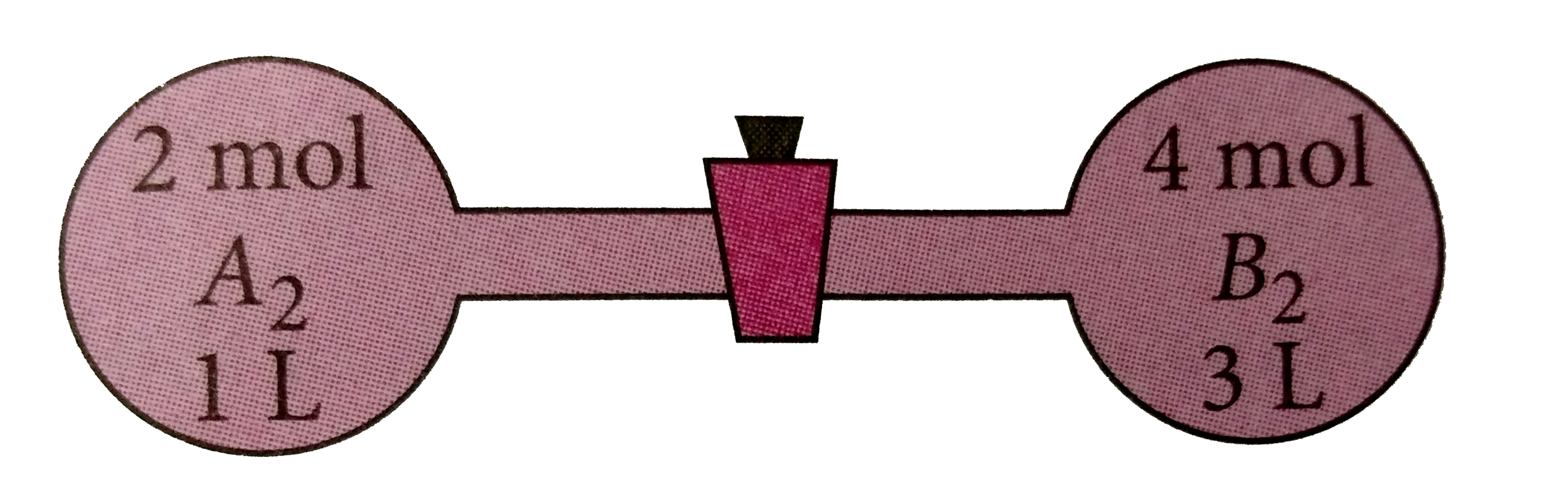
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- When A(2) and B(2) are allowed to react, the equilibrium constant of t...
Text Solution
|
- The equilibrium constant for the reaction A(2)(g)+B(2)(g) hArr 2AB(g) ...
Text Solution
|
- Determinre the value of equilibrium constant (K(C)) for the reaction ...
Text Solution
|
- Calculate the equilibrium constant (K(c)) for the reaction given belo...
Text Solution
|
- The gas A(2) in the left flask allowed to react with gas B(2) present ...
Text Solution
|
- The equilibrium constant (K(c)) of the reaction A(2)(g) + B(2)(g) rarr...
Text Solution
|
- 10 moles of A(2), 15 moles of B(2) and 5 moles of AB are placed in a 2...
Text Solution
|
- When A(2) and B(2) are allowed to react, the equilibrium constant of t...
Text Solution
|
- For the Chemical reaction A(2(g)) + B(2(g)) hArr 2 AB(g) the amount o...
Text Solution
|