A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- FeCl(3) on reaction with K(4)[fe(CN)(6)] in aq. Solution gives blue co...
Text Solution
|
- FeCl(3) on reaction with K(4)[Fe(CN)(6)] in aqueous solution gives blu...
Text Solution
|
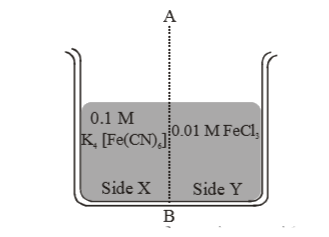
- Two solution (A) containing FeCl(3(aq.)) and separated by semipermeabl...
Text Solution
|
- Two solution (A)containing Fecl(3)(aq) and(B) semipermeable membrance ...
Text Solution
|
- FeCI(3) on reaction with K(4)[Fe(CN)(6)] in aqueous solution gives blu...
Text Solution
|
- F(3)CI(3) on reaction with K(4)[Fe(CN)(6)] in aqueous solution gives b...
Text Solution
|
- Solution (A) containing FeCl(3) is separated from solution (B) contain...
Text Solution
|
- CNS^(-) ions give red colour with Fe^(3+) ions in aqueous solution as:...
Text Solution
|
- Which one of the following statement "(s)" is/are correct regarding P...
Text Solution
|