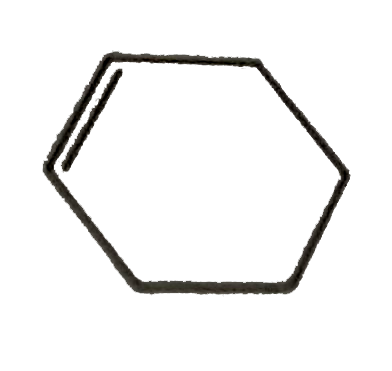
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- If enthalpy of hydrogenation of C(6)H(6)(l) into C(6)H(12)(l) is -205k...
Text Solution
|
- Mole fraction of I(2) in C(6) H(6) is 0.2. Calculate molality of I(2) ...
Text Solution
|
- alpha- maltose (C(12)H(22)O(11)) can be hydrolysed to glucose (C(6)H(1...
Text Solution
|
- If enthaopy of hydrogenation of C(6)H(6)(l) into C(6)H(12)(l) is -205k...
Text Solution
|
- If enthalpy of hydrogenation of C(6)H(6)(l) into C(6)H(12)(l) is -205 ...
Text Solution
|
- Calculate the DeltaH(f)^@ of C(6)H(12)O(6) (s) from the following data...
Text Solution
|
- The standard enthalpies of combustion of C(6)H(6(l)),C(("graphite"))an...
Text Solution
|
- The standard enthalpies of combustion of C(6)H(6) (l) ,C ( graphite) a...
Text Solution
|
- Covert C(6)H(6) to C(6)H(5)D
Text Solution
|