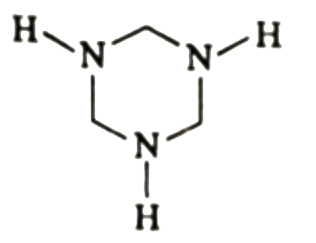
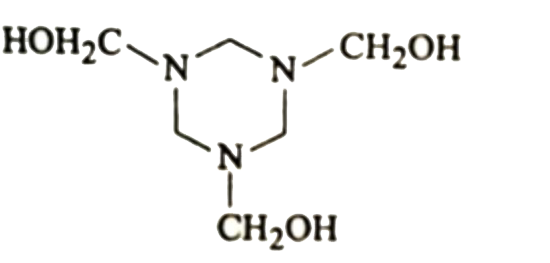
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Formaldehyde reacts with excess of ammonia to give
Text Solution
|
- मेथेनल या फॉर्मेल्डिहाइड अमोनिया के साथ अभिक्रिया करता है ?
Text Solution
|
- फॉर्मेल्डिहाइड अमोनिया के साथ क्रिया करके ……… बनाता है, जो ……. के नाम ...
Text Solution
|
- Ammonia reacts with excess of chlorine to give
Text Solution
|
- Formaldehyde reacts with ammonia to give :-
Text Solution
|
- Ethyl iodide reacts with excess ammonia to give
Text Solution
|
- Formaldehyde reacts with excess of ammonia to give
Text Solution
|
- Excess of bromo ethane reacts with alcholic ammonia to give,
Text Solution
|
- Formaldehyde reacts with ammonia to give
Text Solution
|