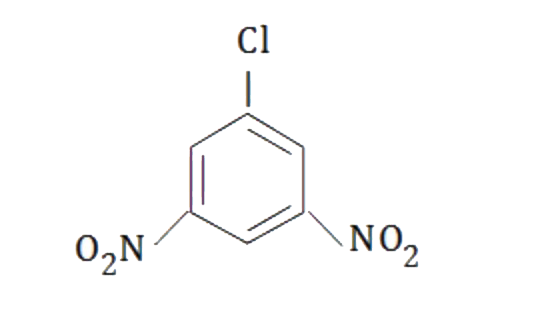
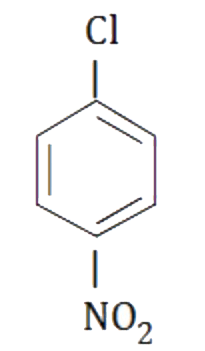
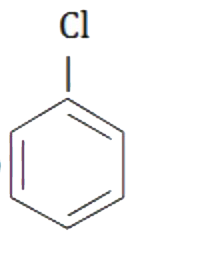
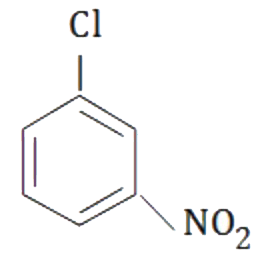
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the aromatic compounds reacts fastest with methoxide ion?
Text Solution
|
- Which of the following reacts the fastest in electrophilic aromatic su...
Text Solution
|
- Which of the following aromatic compounds will react with KMnO(4)?
Text Solution
|
- Which one of the following compounds undergoes bromination of its arom...
Text Solution
|
- Which of the following compounds reacts fastest with Lucas reagent?
Text Solution
|
- Which of the following compound react fastest with sodium metal.
Text Solution
|
- The compound that reacts the fastest with sodium methoxide is
Text Solution
|
- Which of the aromatic compounds reacts fastest with methoxide ion?
Text Solution
|
- Which of the aromatic compounds reacts fastest with methoxide ion?
Text Solution
|