A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
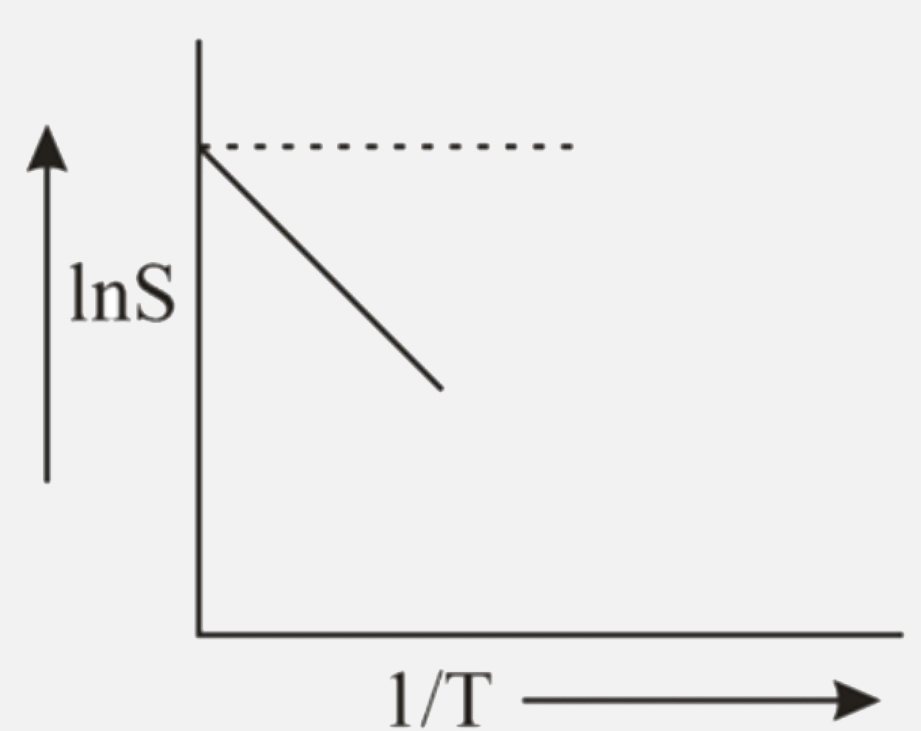
- The solubility (S) of solute in water varies with temperature as given...
Text Solution
|
- Solubility of a solute in water is dependent on temperature as given b...
Text Solution
|
- Solubility (S) of a solution in a solvent (say H(2)) is dependent on ...
Text Solution
|
- The enthalpy of solution of NaOH(s) in water is -41.6 kJ//"mole" When ...
Text Solution
|
- Study the figure given below and mark the correct expression. The...
Text Solution
|
- 1.5 g of solute is dissolved in 15 g of water to form a saturated solu...
Text Solution
|
- solution is one that contains less solute than that of the sa...
Text Solution
|
- The solubility (S) of solute in water varies with temperature as given...
Text Solution
|
- Solubility of a solute in a solvent ( say H(2)O ) is dependent on temp...
Text Solution
|