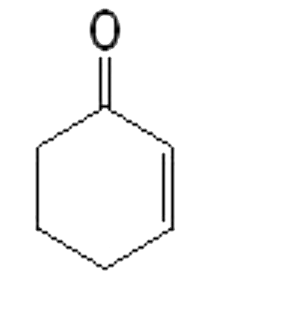
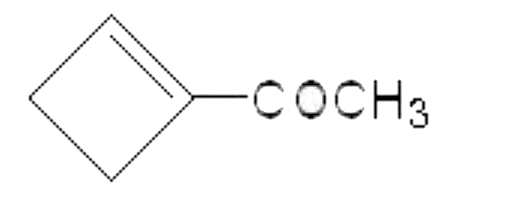
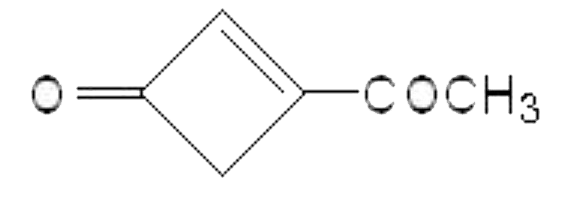
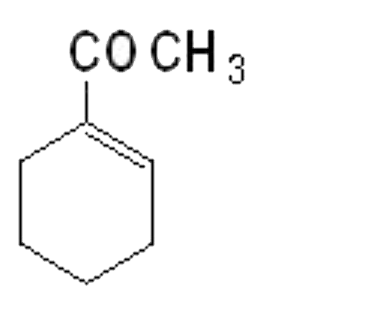
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- OHC(CH(2))(3)COCH(3)overset(OH^(-))underset(Delta)rarr The major pro...
Text Solution
|
- Consider the thermal decomposition CH+(3)CH(2)underset(CH(3))underset(...
Text Solution
|
- The major product formed in the reaction CH(3)-underset(OH)underse...
Text Solution
|
- Identify final product in the following reaction, CH(3)underset(OH)u...
Text Solution
|
- H(3)C-CH(2)-overset(CH(3))overset(|)(CH)-underset(O)underset(||)(C)-NH...
Text Solution
|
- CH(3)-underset(Br)underset(|)CH-CH(2)-overset(O)overset(||)C-CH(3)unde...
Text Solution
|
- CH(3)-underset(OH)underset(|)overset(CH(3))overset(|)(C)-underset(NH(2...
Text Solution
|
- The major product of the following reaction is CH(3)-overset(CH(3))ove...
Text Solution
|
- CH(3)CO(CH(2))(4)CHO underset(Delta)overset(overset(Θ)OH)to major pro...
Text Solution
|