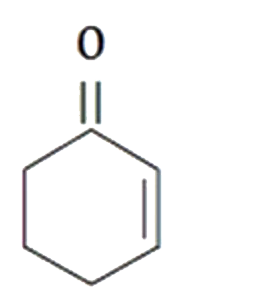
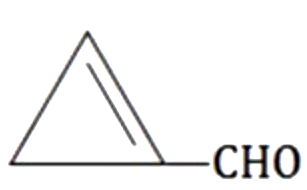
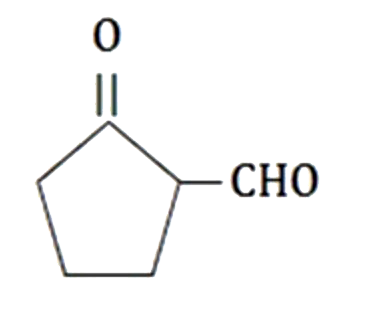
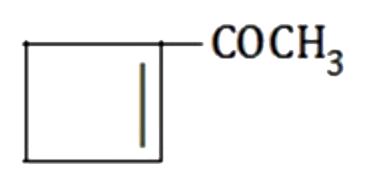
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In the given reaction, OHC-(CH(2))(3)-overset(O)overset(||)C-CH(3)ov...
Text Solution
|
- In the reaction sequence CH(3)-overset(O)overset(||)(C)-Hunderset(over...
Text Solution
|
- CH(3)-overset(O)overset(||)C-CH(3) " and " CH(3)-overset(O)overset(||)...
Text Solution
|
- CH(3)-overset(O)overset(||)C-H " and "CH(3)-overset(O)overset(||)C-CH...
Text Solution
|
- CH(3)-overset(O)overset(||)(C)-O-CH(2)-CH(3)+H-O/^(-)rarr (O/=O^(18)) ...
Text Solution
|
- CH(3)-overset(O)overset(||)(C)-CH(2)-CH(2)-overset(O)overset(||)(C)-CH...
Text Solution
|
- Find the product of following reaction: Ph-CH(2)-overset(O)overset(||)...
Text Solution
|
- Ch(3)-overset(O)overset(||)C-overset(O)overset(||)C-Phoverset(CH(3)CO(...
Text Solution
|
- CH(3)-overset(O)overset(||)C-CH(3)overset(CH(2)N(2))rarr(X)overset(SeO...
Text Solution
|