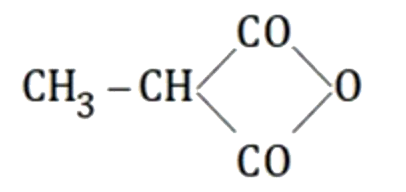
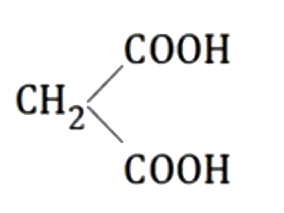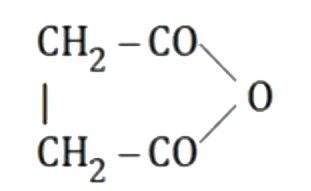A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- In the given reaction CH(3)-COOH underset(underset("(iii) "H(2)O //H^(...
Text Solution
|
- In the given reaction C(6)H(5)-overset(O)overset(||)(C)-CH(3)underse...
Text Solution
|
- In the given reaction CH(3)-COOHundersetunderset((iii)." "H(2)O //H^...
Text Solution
|
- H(3)C-C-=C-underset(CH(3))underset(|)N-CH(3)underset(H(2)O)overset(Hg^...
Text Solution
|
- CH(3)COOH overset(Br(2)//P)rarr Y underset((i) H(3)O^(+))overset((i)KC...
Text Solution
|
- CH(3)CH(2)OH underset(I)overset(Cu.573 K)rarr x underset(II)overset([O...
Text Solution
|
- In the following reaction sequence, CH(3) - underset((X))underset(OH...
Text Solution
|
- Final product of following reaction is CO(2) + (CH(3))(3) C - MgBr und...
Text Solution
|
- अभिक्रियाओं के क्रम CH(3)CH(2)OH underset("क्रोमिक अम्ल")overset([O])r...
Text Solution
|